Abstract
OBJECTIVE—Thrombospondin-1 (TSP-1), a trimeric glycoprotein, is involved in cell-matrix interactions of various tissues, particularly in cartilage. Biochemical analyses show expression of TSP-1 in human cartilage, but its cellular source as well as the presence of its main surface receptors CD36 and CD51 in normal and osteoarthritic cartilage remain unknown. Therefore, to localise TSP-1 and its receptors immunohistochemistry and in situ hybridisation were used. METHODS—Radioactive in situ hybridisations with an RNA probe that encodes TSP-1 combined with immunostaining were carried out to investigate the expression patterns of TSP-1, CD36, and CD51 in seven normal and 23 osteoarthritic human cartilage samples. RESULTS—In normal cartilage TSP-1 was present mainly in the middle and upper deep zone. RNA expression was predominantly seen over chondrocytes of the middle zone. CD36 was found in chondrocytes of the superficial and upper middle zone. In mild and moderate osteoarthritic cartilage an increased number of TSP-1 expressing chondrocytes were seen and an increased pericellular staining close to the surface. In severe osteoarthritic cartilage a decrease in the number of TSP-1 synthesising chondrocytes and a strong reduction in matrix staining were observed. Most of these severe osteoarthritic samples showed a strongly enhanced number of CD36 positive chondrocytes. CONCLUSION—The cellular source of TSP-1 in normal cartilage is mainly mid-zone chondrocytes, which also express CD36. In early osteoarthritic cartilage lesions an increase of TSP-1 was seen, whereas reduced TSP-1 synthesis is paralleled by a strong decrease in TSP-1 protein staining in severe osteoarthritis. Furthermore, in severe osteoarthritic cartilage the number of CD36 immunostained chondrocytes is significantly increased.
Full Text
The Full Text of this article is available as a PDF (247.9 KB).
Figure 1 .
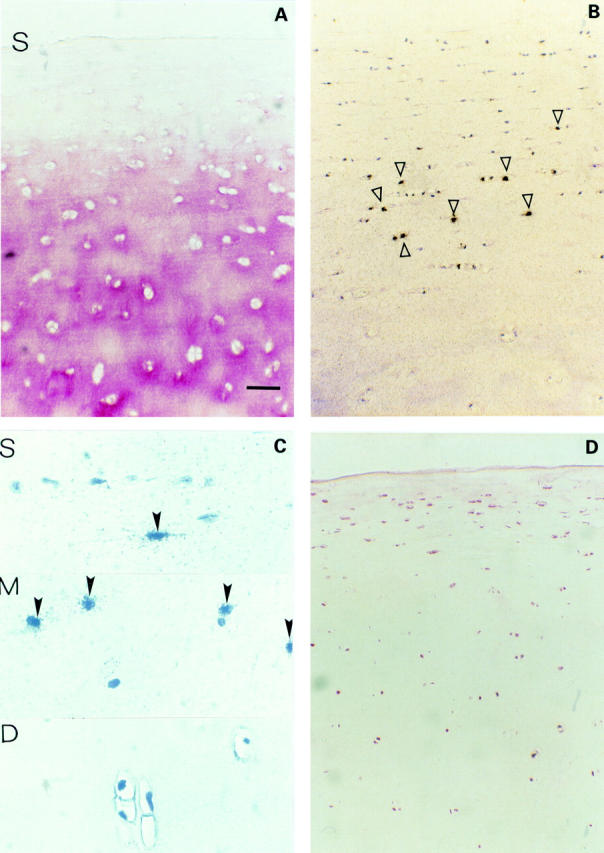
(A) Normal cartilage (three of seven samples, Mankin 1 and 0). Immunohistochemical staining of TSP-1 in normal human cartilage without TSP-1 staining in the superficial zone (S indicates cartilage surface). (B) In situ hybridisation of TSP-1 shows strong expression mainly in middle zone chondrocytes (open arrowheads). (C) High power magnification of typical zonal areas of the superficial (S), middle (M) and the deep (D) zone with positive TSP-1 RNA signals over chondrocytes of the superficial and middle zones (closed arrowheads). (D) Typical distribution of CD36 immunostained chondrocytes in normal cartilage. Bar represents 115 µm in figs A, B, and D and 30 µm in fig C.
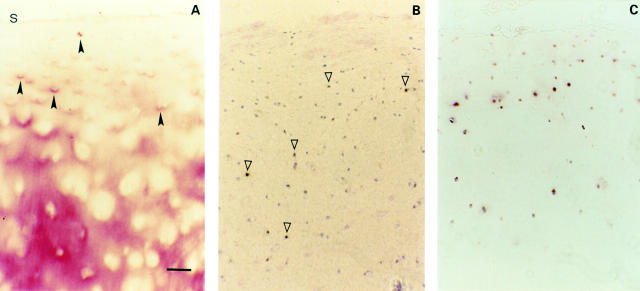
Figure 2 .
(A) Mild OA cartilage (one of seven samples, Mankin 4). Immunohistochemical staining of TSP-1 in mild OA cartilage showing chondrocytes with pericellular TSP-1 staining in the upper middle zone (closed arrowheads) and a predominantly interterritorial staining in the deep zone (s indicates cartilage surface). (B) In situ hybridisation of TSP-1 shows expression of middle and deep zone chondrocytes (open arrowheads). (C) CD36 positive chondrocytes are present in the middle and upper deep zones. Bar represents 125 µm in figs A, B, and C.
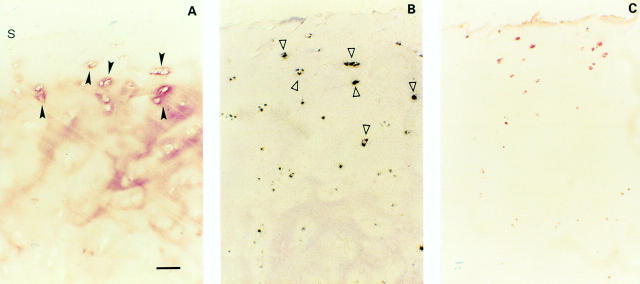
Figure 3 .
(A) Moderate OA cartilage (one of seven samples, Mankin 7). TSP-1 protein in moderate OA cartilage is present in the upper regions with a pericellular distribution, whereas in the deeper regions a slight interterritorial staining persists (s indicates cartilage surface). (B) Strong TSP-1 expression of the pericellularly stained chondrocytes was found by in situ hybridisation. (C) CD36 positive chondrocytes in co-localisation with TSP-1 expressing chondrocytes. Bar represents 125 µm in figs A, B, and C.
Figure 4 .
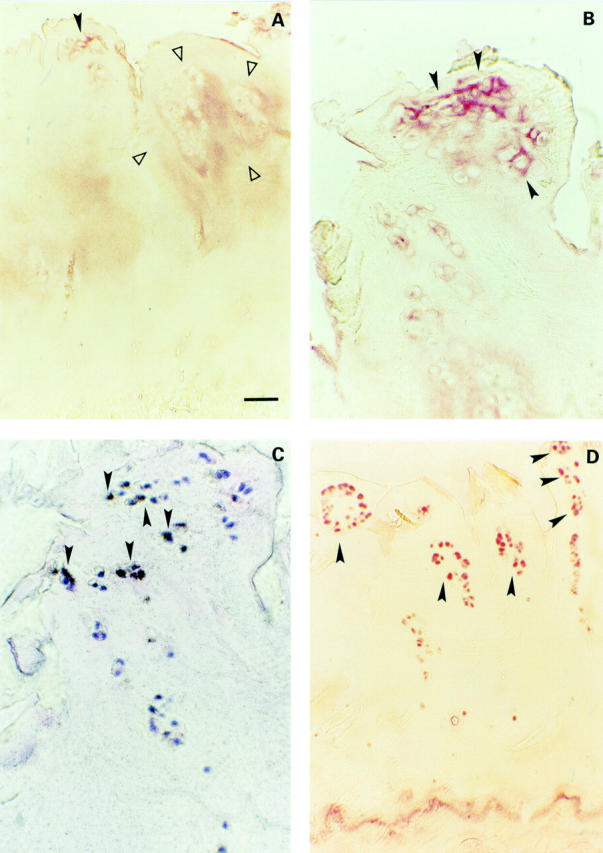
(A) Severe OA cartilage (two of nine samples, Mankin 13 and 14). Weak TSP-1 staining in the pericellular matrix of clustered chondrocytes (closed arrowhead) and a weak staining territorial and interterritorial on the right side (area between open arrowheads). (B) Magnification of a large chondrocyte cluster with a pericellular TSP-1 staining (closed arrowheads). (C) Adjacent slide with clustered chondrocytes showing TSP-1 expression (closed arrowheads). (D) Strong CD-36 protein expression of nearly 90% of the remaining clustered chondrocytes (closed arrowheads). Bar represents 125 µm in figs A and D, and 50 µm in B and C.
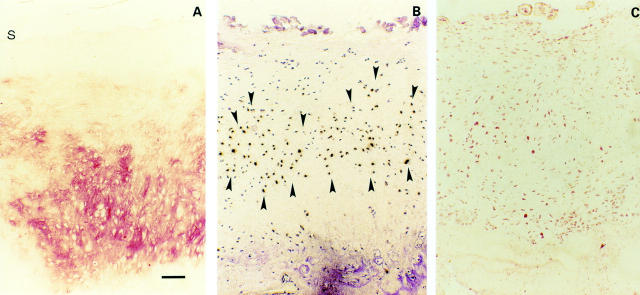
Figure 5 .
(A) Osteophytes. Strong immunohistochemical TSP-1 staining in osteophytes (s indicates cartilage surface). (B) Paralleled by a strong TSP-1 expression by in situ hybridisation (area between closed arrowheads). (C) Most of the chondrocytes are CD36 positive. Bar represents 125 µm in figs A, B, and C.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aigner T., Bertling W., Stöss H., Weseloh G., von der Mark K. Independent expression of fibril-forming collagens I, II, and III in chondrocytes of human osteoarthritic cartilage. J Clin Invest. 1993 Mar;91(3):829–837. doi: 10.1172/JCI116303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawford S. E., Stellmach V., Murphy-Ullrich J. E., Ribeiro S. M., Lawler J., Hynes R. O., Boivin G. P., Bouck N. Thrombospondin-1 is a major activator of TGF-beta1 in vivo. Cell. 1998 Jun 26;93(7):1159–1170. doi: 10.1016/s0092-8674(00)81460-9. [DOI] [PubMed] [Google Scholar]
- DiCesare P. E., Mörgelin M., Mann K., Paulsson M. Cartilage oligomeric matrix protein and thrombospondin 1. Purification from articular cartilage, electron microscopic structure, and chondrocyte binding. Eur J Biochem. 1994 Aug 1;223(3):927–937. doi: 10.1111/j.1432-1033.1994.tb19070.x. [DOI] [PubMed] [Google Scholar]
- Hardingham T. Proteoglycans: their structure, interactions and molecular organization in cartilage. Biochem Soc Trans. 1981 Dec;9(6):489–497. doi: 10.1042/bst0090489. [DOI] [PubMed] [Google Scholar]
- Heinegård D., Oldberg A. Structure and biology of cartilage and bone matrix noncollagenous macromolecules. FASEB J. 1989 Jul;3(9):2042–2051. doi: 10.1096/fasebj.3.9.2663581. [DOI] [PubMed] [Google Scholar]
- Herbst H., Frey A., Heinrichs O., Milani S., Bechstein W. O., Neuhaus P., Schuppan D. Heterogeneity of liver cells expressing procollagen types I and IV in vivo. Histochem Cell Biol. 1997 May;107(5):399–409. doi: 10.1007/s004180050126. [DOI] [PubMed] [Google Scholar]
- Iruela-Arispe M. L., Liska D. J., Sage E. H., Bornstein P. Differential expression of thrombospondin 1, 2, and 3 during murine development. Dev Dyn. 1993 May;197(1):40–56. doi: 10.1002/aja.1001970105. [DOI] [PubMed] [Google Scholar]
- Lamszus K., Joseph A., Jin L., Yao Y., Chowdhury S., Fuchs A., Polverini P. J., Goldberg I. D., Rosen E. M. Scatter factor binds to thrombospondin and other extracellular matrix components. Am J Pathol. 1996 Sep;149(3):805–819. [PMC free article] [PubMed] [Google Scholar]
- Lawler J., Hynes R. O. The structure of human thrombospondin, an adhesive glycoprotein with multiple calcium-binding sites and homologies with several different proteins. J Cell Biol. 1986 Nov;103(5):1635–1648. doi: 10.1083/jcb.103.5.1635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lohmander L. S. Articular cartilage and osteoarthrosis. The role of molecular markers to monitor breakdown, repair and disease. J Anat. 1994 Jun;184(Pt 3):477–492. [PMC free article] [PubMed] [Google Scholar]
- Lyons-Giordano B., Kefalides N. A., Brinker J. M., Pratta M. A., Arner E. C. The effects of interleukin-1 on the expression of thrombospondin and fibronectin by rabbit articular chondrocytes. Exp Cell Res. 1991 Aug;195(2):462–467. doi: 10.1016/0014-4827(91)90397-d. [DOI] [PubMed] [Google Scholar]
- Mankin H. J., Dorfman H., Lippiello L., Zarins A. Biochemical and metabolic abnormalities in articular cartilage from osteo-arthritic human hips. II. Correlation of morphology with biochemical and metabolic data. J Bone Joint Surg Am. 1971 Apr;53(3):523–537. [PubMed] [Google Scholar]
- Mayne R. Cartilage collagens. What is their function, and are they involved in articular disease? Arthritis Rheum. 1989 Mar;32(3):241–246. doi: 10.1002/anr.1780320302. [DOI] [PubMed] [Google Scholar]
- Milani S., Herbst H., Schuppan D., Hahn E. G., Stein H. In situ hybridization for procollagen types I, III and IV mRNA in normal and fibrotic rat liver: evidence for predominant expression in nonparenchymal liver cells. Hepatology. 1989 Jul;10(1):84–92. doi: 10.1002/hep.1840100117. [DOI] [PubMed] [Google Scholar]
- Miller R. R., McDevitt C. A. Thrombospondin 1 binds to the surface of bovine articular chondrocytes by a linear RGD-dependent mechanism. FEBS Lett. 1995 Apr 24;363(3):214–216. doi: 10.1016/0014-5793(95)00311-v. [DOI] [PubMed] [Google Scholar]
- Miller R. R., McDevitt C. A. Thrombospondin is present in articular cartilage and is synthesized by articular chondrocytes. Biochem Biophys Res Commun. 1988 Jun 16;153(2):708–714. doi: 10.1016/s0006-291x(88)81152-5. [DOI] [PubMed] [Google Scholar]
- Moos V., Fickert S., Müller B., Weber U., Sieper J. Immunohistological analysis of cytokine expression in human osteoarthritic and healthy cartilage. J Rheumatol. 1999 Apr;26(4):870–879. [PubMed] [Google Scholar]
- Pfander D., Cramer T., Weseloh G., Pullig O., Schuppan D., Bauer M., Swoboda B. Hepatocyte growth factor in human osteoarthritic cartilage. Osteoarthritis Cartilage. 1999 Nov;7(6):548–559. doi: 10.1053/joca.1999.0259. [DOI] [PubMed] [Google Scholar]
- Pfander D., Rahmanzadeh R., Scheller E. E. Presence and distribution of collagen II, collagen I, fibronectin, and tenascin in rabbit normal and osteoarthritic cartilage. J Rheumatol. 1999 Feb;26(2):386–394. [PubMed] [Google Scholar]
- Qian X., Tuszynski G. P. Expression of thrombospondin-1 in cancer: a role in tumor progression. Proc Soc Exp Biol Med. 1996 Jul;212(3):199–207. doi: 10.3181/00379727-212-44008. [DOI] [PubMed] [Google Scholar]
- Swoboda B., Pullig O., Kirsch T., Kladny B., Steinhäuser B., Weseloh G. Increased content of type-VI collagen epitopes in human osteoarthritic cartilage: quantitation by inhibition ELISA. J Orthop Res. 1998 Jan;16(1):96–99. doi: 10.1002/jor.1100160116. [DOI] [PubMed] [Google Scholar]
- Tucker R. P., Hagios C., Chiquet-Ehrismann R., Lawler J. In situ localization of thrombospondin-1 and thrombospondin-3 transcripts in the avian embryo. Dev Dyn. 1997 Mar;208(3):326–337. doi: 10.1002/(SICI)1097-0177(199703)208:3<326::AID-AJA4>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- Urban J. P. The chondrocyte: a cell under pressure. Br J Rheumatol. 1994 Oct;33(10):901–908. doi: 10.1093/rheumatology/33.10.901. [DOI] [PubMed] [Google Scholar]