Abstract
OBJECTIVE—Matrix metalloproteinases (MMPs) are expressed in joint tissues of patients with rheumatoid arthritis (RA) and osteoarthritis (OA). The objective of this study was to define the steady state levels of seven different MMPs and two tissue inhibitors of metalloproteinases (TIMPs) as well as the potential metalloproteinase activity in the synovial fluid (SF) to provide more insight into the role of MMPs in cartilage destruction in RA and OA. METHODS—Levels of MMP-1, MMP-2, MMP-3, MMP-7, MMP-8, MMP-9, MMP-13, TIMP-1, and TIMP-2 in SF aspirated from knee joints of 97 patients with RA and 103 patients with OA were measured by the corresponding one step sandwich enzyme immunoassays. Proteolytic activity of MMPs in these SFs was examined in an assay using [3H]carboxymethylated transferrin substrate in the presence of inhibitors of serine and cysteine proteinases after activation with p-aminophenylmercuric acetate (APMA). Destruction of RA knee joints was radiographically evaluated. RESULTS—Levels of MMP-1, MMP-2, MMP-3, MMP-8, and MMP-9 were significantly higher in RA SF than in OA SF. MMP-7 and MMP-13 were detectable in more than 45% of RA SFs and in less than 20% of OA SFs, respectively. Among the MMPs examined, MMP-3 levels were extremely high compared with those of other MMPs. Direct correlations were seen between the levels of MMP-1 and MMP-3 and between those of MMP-8 and MMP-9 in RA SF. Although the levels of MMP-1 and MMP-3 increased even in the early stage of RA, those of MMP-8 and MMP-9 were low in the early stage and increased with the progression of RA. Molar ratios of the total amounts of the MMPs to those of the TIMPs were 5.2-fold higher in patients with RA than in OA, which was significant. APMA-activated metalloproteinase activity in SF showed a similar result, and a direct correlation was seen between the molar ratios and the activity in RA SF. CONCLUSIONS—Our results show that high levels of MMP-1, MMP-2, MMP-3, MMP-8, MMP-9, and TIMP-1 are present in RA SF and suggest that once these MMPs are fully activated, they have an imbalance against TIMPs, which may contribute to the cartilage destruction in RA.
Full Text
The Full Text of this article is available as a PDF (162.1 KB).
Figure 1 .
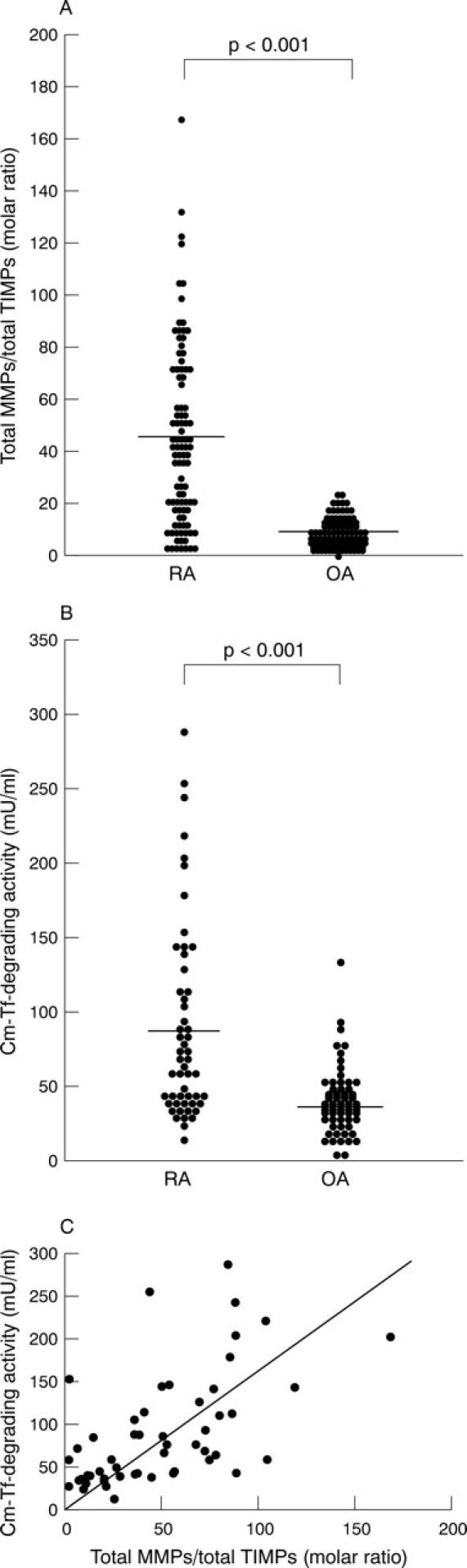
Molar ratios of matrix metalloproteinases (MMPs) to tissue inhibitors of metalloproteinases (TIMPs), carboxymethylated transferrin (Cm-Tf)-degrading activity, and correlation between the molar ratios and the activity. (A) Molar ratios of total MMPs to total TIMPs were calculated as described in "Materials and methods". Bars indicate mean values in RA and OA synovial fluids (SFs). (B) Cm-Tf-degrading activity in SF was measured after the p-aminophenylmercuric acetate (APMA) activation in the presence of serine and cysteine proteinase inhibitors as described in "Materials and methods". Bars indicate mean values in RA and OA SFs. (C) Correlation between the molar ratios and the Cm-Tf-degrading activity. Note a significant direct correlation (rs=0.580, p<0.001).
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ahrens D., Koch A. E., Pope R. M., Stein-Picarella M., Niedbala M. J. Expression of matrix metalloproteinase 9 (96-kd gelatinase B) in human rheumatoid arthritis. Arthritis Rheum. 1996 Sep;39(9):1576–1587. doi: 10.1002/art.1780390919. [DOI] [PubMed] [Google Scholar]
- Altman R., Asch E., Bloch D., Bole G., Borenstein D., Brandt K., Christy W., Cooke T. D., Greenwald R., Hochberg M. Development of criteria for the classification and reporting of osteoarthritis. Classification of osteoarthritis of the knee. Diagnostic and Therapeutic Criteria Committee of the American Rheumatism Association. Arthritis Rheum. 1986 Aug;29(8):1039–1049. doi: 10.1002/art.1780290816. [DOI] [PubMed] [Google Scholar]
- Arnett F. C., Edworthy S. M., Bloch D. A., McShane D. J., Fries J. F., Cooper N. S., Healey L. A., Kaplan S. R., Liang M. H., Luthra H. S. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum. 1988 Mar;31(3):315–324. doi: 10.1002/art.1780310302. [DOI] [PubMed] [Google Scholar]
- Bartlett J. D., Simmer J. P., Xue J., Margolis H. C., Moreno E. C. Molecular cloning and mRNA tissue distribution of a novel matrix metalloproteinase isolated from porcine enamel organ. Gene. 1996 Dec 12;183(1-2):123–128. doi: 10.1016/s0378-1119(96)00525-2. [DOI] [PubMed] [Google Scholar]
- Beekman B., van El B., Drijfhout J. W., Ronday H. K., TeKoppele J. M. Highly increased levels of active stromelysin in rheumatoid synovial fluid determined by a selective fluorogenic assay. FEBS Lett. 1997 Dec 1;418(3):305–309. doi: 10.1016/s0014-5793(97)01371-9. [DOI] [PubMed] [Google Scholar]
- Birkedal-Hansen H., Moore W. G., Bodden M. K., Windsor L. J., Birkedal-Hansen B., DeCarlo A., Engler J. A. Matrix metalloproteinases: a review. Crit Rev Oral Biol Med. 1993;4(2):197–250. doi: 10.1177/10454411930040020401. [DOI] [PubMed] [Google Scholar]
- Cawston T. E., McLaughlin P., Hazleman B. L. Paired serum and synovial fluid values of alpha 2-macroglobulin and TIMP in rheumatoid arthritis. Br J Rheumatol. 1987 Oct;26(5):354–358. doi: 10.1093/rheumatology/26.5.354. [DOI] [PubMed] [Google Scholar]
- Chatham W. W., Heck L. W., Blackburn W. D., Jr Ligand-dependent release of active neutrophil collagenase. Arthritis Rheum. 1990 Feb;33(2):228–234. doi: 10.1002/art.1780330211. [DOI] [PubMed] [Google Scholar]
- Clark I. M., Powell L. K., Ramsey S., Hazleman B. L., Cawston T. E. The measurement of collagenase, tissue inhibitor of metalloproteinases (TIMP), and collagenase-TIMP complex in synovial fluids from patients with osteoarthritis and rheumatoid arthritis. Arthritis Rheum. 1993 Mar;36(3):372–379. doi: 10.1002/art.1780360313. [DOI] [PubMed] [Google Scholar]
- Cole A. A., Chubinskaya S., Schumacher B., Huch K., Szabo G., Yao J., Mikecz K., Hasty K. A., Kuettner K. E. Chondrocyte matrix metalloproteinase-8. Human articular chondrocytes express neutrophil collagenase. J Biol Chem. 1996 May 3;271(18):11023–11026. doi: 10.1074/jbc.271.18.11023. [DOI] [PubMed] [Google Scholar]
- Cooper T. W., Eisen A. Z., Stricklin G. P., Welgus H. G. Platelet-derived collagenase inhibitor: characterization and subcellular localization. Proc Natl Acad Sci U S A. 1985 May;82(9):2779–2783. doi: 10.1073/pnas.82.9.2779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiBattista J. A., Pelletier J. P., Zafarullah M., Fujimoto N., Obata K., Martel-Pelletier J. Coordinate regulation of matrix metalloproteases and tissue inhibitor of metalloproteinase expression in human synovial fibroblasts. J Rheumatol Suppl. 1995 Feb;43:123–128. [PubMed] [Google Scholar]
- Farr M., Wainwright A., Salmon M., Hollywell C. A., Bacon P. A. Platelets in the synovial fluid of patients with rheumatoid arthritis. Rheumatol Int. 1984;4(1):13–17. doi: 10.1007/BF00683878. [DOI] [PubMed] [Google Scholar]
- Frisch S. M., Clark E. J., Werb Z. Coordinate regulation of stromelysin and collagenase genes determined with cDNA probes. Proc Natl Acad Sci U S A. 1987 May;84(9):2600–2604. doi: 10.1073/pnas.84.9.2600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujimoto N., Hosokawa N., Iwata K., Shinya T., Okada Y., Hayakawa T. A one-step sandwich enzyme immunoassay for inactive precursor and complexed forms of human matrix metalloproteinase 9 (92 kDa gelatinase/type IV collagenase, gelatinase B) using monoclonal antibodies. Clin Chim Acta. 1994 Nov;231(1):79–88. doi: 10.1016/0009-8981(94)90256-9. [DOI] [PubMed] [Google Scholar]
- Fujimoto N., Mouri N., Iwata K., Ohuchi E., Okada Y., Hayakawa T. A one-step sandwich enzyme immunoassay for human matrix metalloproteinase 2 (72-kDa gelatinase/type IV collagenase) using monoclonal antibodies. Clin Chim Acta. 1993 Nov 30;221(1-2):91–103. doi: 10.1016/0009-8981(93)90024-x. [DOI] [PubMed] [Google Scholar]
- Fujimoto N., Zhang J., Iwata K., Shinya T., Okada Y., Hayakawa T. A one-step sandwich enzyme immunoassay for tissue inhibitor of metalloproteinases-2 using monoclonal antibodies. Clin Chim Acta. 1993 Oct 29;220(1):31–45. doi: 10.1016/0009-8981(93)90004-n. [DOI] [PubMed] [Google Scholar]
- Gravallese E. M., Darling J. M., Ladd A. L., Katz J. N., Glimcher L. H. In situ hybridization studies of stromelysin and collagenase messenger RNA expression in rheumatoid synovium. Arthritis Rheum. 1991 Sep;34(9):1076–1084. doi: 10.1002/art.1780340903. [DOI] [PubMed] [Google Scholar]
- Greene J., Wang M., Liu Y. E., Raymond L. A., Rosen C., Shi Y. E. Molecular cloning and characterization of human tissue inhibitor of metalloproteinase 4. J Biol Chem. 1996 Nov 29;271(48):30375–30380. doi: 10.1074/jbc.271.48.30375. [DOI] [PubMed] [Google Scholar]
- Grillet B., Dequeker J., Paemen L., Van Damme B., Opdenakker G. Gelatinase B in chronic synovitis: immunolocalization with a monoclonal antibody. Br J Rheumatol. 1997 Jul;36(7):744–747. doi: 10.1093/rheumatology/36.7.744. [DOI] [PubMed] [Google Scholar]
- Hanemaaijer R., Sorsa T., Konttinen Y. T., Ding Y., Sutinen M., Visser H., van Hinsbergh V. W., Helaakoski T., Kainulainen T., Rönkä H. Matrix metalloproteinase-8 is expressed in rheumatoid synovial fibroblasts and endothelial cells. Regulation by tumor necrosis factor-alpha and doxycycline. J Biol Chem. 1997 Dec 12;272(50):31504–31509. doi: 10.1074/jbc.272.50.31504. [DOI] [PubMed] [Google Scholar]
- Hembry R. M., Bagga M. R., Reynolds J. J., Hamblen D. L. Immunolocalisation studies on six matrix metalloproteinases and their inhibitors, TIMP-1 and TIMP-2, in synovia from patients with osteo- and rheumatoid arthritis. Ann Rheum Dis. 1995 Jan;54(1):25–32. doi: 10.1136/ard.54.1.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kodama S., Iwata K., Iwata H., Yamashita K., Hayakawa T. Rapid one-step sandwich enzyme immunoassay for tissue inhibitor of metalloproteinases. An application for rheumatoid arthritis serum and plasma. J Immunol Methods. 1990 Feb 20;127(1):103–108. doi: 10.1016/0022-1759(90)90345-v. [DOI] [PubMed] [Google Scholar]
- Larsen A., Dale K., Eek M. Radiographic evaluation of rheumatoid arthritis and related conditions by standard reference films. Acta Radiol Diagn (Stockh) 1977 Jul;18(4):481–491. doi: 10.1177/028418517701800415. [DOI] [PubMed] [Google Scholar]
- Matsuki H., Fujimoto N., Iwata K., Knäuper V., Okada Y., Hayakawa T. A one-step sandwich enzyme immunoassay for human matrix metalloproteinase 8 (neutrophil collagenase) using monoclonal antibodies. Clin Chim Acta. 1996 Jan 31;244(2):129–143. doi: 10.1016/0009-8981(95)06197-5. [DOI] [PubMed] [Google Scholar]
- McCachren S. S. Expression of metalloproteinases and metalloproteinase inhibitor in human arthritic synovium. Arthritis Rheum. 1991 Sep;34(9):1085–1093. doi: 10.1002/art.1780340904. [DOI] [PubMed] [Google Scholar]
- Mohtai M., Smith R. L., Schurman D. J., Tsuji Y., Torti F. M., Hutchinson N. I., Stetler-Stevenson W. G., Goldberg G. I. Expression of 92-kD type IV collagenase/gelatinase (gelatinase B) in osteoarthritic cartilage and its induction in normal human articular cartilage by interleukin 1. J Clin Invest. 1993 Jul;92(1):179–185. doi: 10.1172/JCI116547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mudgett J. S., Hutchinson N. I., Chartrain N. A., Forsyth A. J., McDonnell J., Singer I. I., Bayne E. K., Flanagan J., Kawka D., Shen C. F. Susceptibility of stromelysin 1-deficient mice to collagen-induced arthritis and cartilage destruction. Arthritis Rheum. 1998 Jan;41(1):110–121. doi: 10.1002/1529-0131(199801)41:1<110::AID-ART14>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- Nagase H. Activation mechanisms of matrix metalloproteinases. Biol Chem. 1997 Mar-Apr;378(3-4):151–160. [PubMed] [Google Scholar]
- Nguyen Q., Mort J. S., Roughley P. J. Preferential mRNA expression of prostromelysin relative to procollagenase and in situ localization in human articular cartilage. J Clin Invest. 1992 Apr;89(4):1189–1197. doi: 10.1172/JCI115702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohta S., Imai K., Yamashita K., Matsumoto T., Azumano I., Okada Y. Expression of matrix metalloproteinase 7 (matrilysin) in human osteoarthritic cartilage. Lab Invest. 1998 Jan;78(1):79–87. [PubMed] [Google Scholar]
- Ohuchi E., Azumano I., Yoshida S., Iwata K., Okada Y. A one-step sandwich enzyme immunoassay for human matrix metalloproteinase 7 (matrilysin) using monoclonal antibodies. Clin Chim Acta. 1996 Jan 31;244(2):181–198. doi: 10.1016/0009-8981(95)06199-1. [DOI] [PubMed] [Google Scholar]
- Okada Y., Gonoji Y., Nakanishi I., Nagase H., Hayakawa T. Immunohistochemical demonstration of collagenase and tissue inhibitor of metalloproteinases (TIMP) in synovial lining cells of rheumatoid synovium. Virchows Arch B Cell Pathol Incl Mol Pathol. 1990;59(5):305–312. doi: 10.1007/BF02899418. [DOI] [PubMed] [Google Scholar]
- Okada Y., Nagase H., Harris E. D., Jr A metalloproteinase from human rheumatoid synovial fibroblasts that digests connective tissue matrix components. Purification and characterization. J Biol Chem. 1986 Oct 25;261(30):14245–14255. [PubMed] [Google Scholar]
- Okada Y., Shinmei M., Tanaka O., Naka K., Kimura A., Nakanishi I., Bayliss M. T., Iwata K., Nagase H. Localization of matrix metalloproteinase 3 (stromelysin) in osteoarthritic cartilage and synovium. Lab Invest. 1992 Jun;66(6):680–690. [PubMed] [Google Scholar]
- Okada Y., Takeuchi N., Tomita K., Nakanishi I., Nagase H. Immunolocalization of matrix metalloproteinase 3 (stromelysin) in rheumatoid synovioblasts (B cells): correlation with rheumatoid arthritis. Ann Rheum Dis. 1989 Aug;48(8):645–653. doi: 10.1136/ard.48.8.645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pei D. Identification and characterization of the fifth membrane-type matrix metalloproteinase MT5-MMP. J Biol Chem. 1999 Mar 26;274(13):8925–8932. doi: 10.1074/jbc.274.13.8925. [DOI] [PubMed] [Google Scholar]
- Pendás A. M., Knäuper V., Puente X. S., Llano E., Mattei M. G., Apte S., Murphy G., López-Otín C. Identification and characterization of a novel human matrix metalloproteinase with unique structural characteristics, chromosomal location, and tissue distribution. J Biol Chem. 1997 Feb 14;272(7):4281–4286. doi: 10.1074/jbc.272.7.4281. [DOI] [PubMed] [Google Scholar]
- Puente X. S., Pendás A. M., Llano E., Velasco G., López-Otín C. Molecular cloning of a novel membrane-type matrix metalloproteinase from a human breast carcinoma. Cancer Res. 1996 Mar 1;56(5):944–949. [PubMed] [Google Scholar]
- Reboul P., Pelletier J. P., Tardif G., Cloutier J. M., Martel-Pelletier J. The new collagenase, collagenase-3, is expressed and synthesized by human chondrocytes but not by synoviocytes. A role in osteoarthritis. J Clin Invest. 1996 May 1;97(9):2011–2019. doi: 10.1172/JCI118636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato H., Takino T., Okada Y., Cao J., Shinagawa A., Yamamoto E., Seiki M. A matrix metalloproteinase expressed on the surface of invasive tumour cells. Nature. 1994 Jul 7;370(6484):61–65. doi: 10.1038/370061a0. [DOI] [PubMed] [Google Scholar]
- Shinmei M., Kobayashi T., Yoshihara Y., Samura A. Significance of the levels of carboxy terminal type II procollagen peptide, chondroitin sulfate isomers, tissue inhibitor of metalloproteinases, and metalloproteinases in osteoarthritis joint fluid. J Rheumatol Suppl. 1995 Feb;43:78–81. [PubMed] [Google Scholar]
- Sopata I., Wize J., Filipowicz-Sosnowska A., Stanisławska-Biernat E., Brzezińska B., Maśliński S. Neutrophil gelatinase levels in plasma and synovial fluid of patients with rheumatic diseases. Rheumatol Int. 1995;15(1):9–14. doi: 10.1007/BF00286763. [DOI] [PubMed] [Google Scholar]
- Stephenson M. L., Goldring M. B., Birkhead J. R., Krane S. M., Rahmsdorf H. J., Angel P. Stimulation of procollagenase synthesis parallels increases in cellular procollagenase mRNA in human articular chondrocytes exposed to recombinant interleukin 1 beta or phorbol ester. Biochem Biophys Res Commun. 1987 Apr 29;144(2):583–590. doi: 10.1016/s0006-291x(87)80006-2. [DOI] [PubMed] [Google Scholar]
- Takino T., Sato H., Shinagawa A., Seiki M. Identification of the second membrane-type matrix metalloproteinase (MT-MMP-2) gene from a human placenta cDNA library. MT-MMPs form a unique membrane-type subclass in the MMP family. J Biol Chem. 1995 Sep 29;270(39):23013–23020. doi: 10.1074/jbc.270.39.23013. [DOI] [PubMed] [Google Scholar]
- Uría J. A., Ferrando A. A., Velasco G., Freije J. M., López-Otín C. Structure and expression in breast tumors of human TIMP-3, a new member of the metalloproteinase inhibitor family. Cancer Res. 1994 Apr 15;54(8):2091–2094. [PubMed] [Google Scholar]
- Velasco G., Pendás A. M., Fueyo A., Knäuper V., Murphy G., López-Otín C. Cloning and characterization of human MMP-23, a new matrix metalloproteinase predominantly expressed in reproductive tissues and lacking conserved domains in other family members. J Biol Chem. 1999 Feb 19;274(8):4570–4576. doi: 10.1074/jbc.274.8.4570. [DOI] [PubMed] [Google Scholar]
- Venembre P. C., Nguyen C. H., Biou D. R., Durand G. M. Changes in the glycoforms of rat alpha-1-acid glycoprotein during experimental polyarthritis. Clin Chim Acta. 1993 Nov 30;221(1-2):59–71. doi: 10.1016/0009-8981(93)90022-v. [DOI] [PubMed] [Google Scholar]
- Walakovits L. A., Moore V. L., Bhardwaj N., Gallick G. S., Lark M. W. Detection of stromelysin and collagenase in synovial fluid from patients with rheumatoid arthritis and posttraumatic knee injury. Arthritis Rheum. 1992 Jan;35(1):35–42. doi: 10.1002/art.1780350106. [DOI] [PubMed] [Google Scholar]
- Wernicke D., Seyfert C., Hinzmann B., Gromnica-Ihle E. Cloning of collagenase 3 from the synovial membrane and its expression in rheumatoid arthritis and osteoarthritis. J Rheumatol. 1996 Apr;23(4):590–595. [PubMed] [Google Scholar]
- Will H., Hinzmann B. cDNA sequence and mRNA tissue distribution of a novel human matrix metalloproteinase with a potential transmembrane segment. Eur J Biochem. 1995 Aug 1;231(3):602–608. doi: 10.1111/j.1432-1033.1995.tb20738.x. [DOI] [PubMed] [Google Scholar]
- Wolfe G. C., MacNaul K. L., Buechel F. F., McDonnell J., Hoerrner L. A., Lark M. W., Moore V. L., Hutchinson N. I. Differential in vivo expression of collagenase messenger RNA in synovium and cartilage. Quantitative comparison with stromelysin messenger RNA levels in human rheumatoid arthritis and osteoarthritis patients and in two animal models of acute inflammatory arthritis. Arthritis Rheum. 1993 Nov;36(11):1540–1547. doi: 10.1002/art.1780361108. [DOI] [PubMed] [Google Scholar]
- Yoshihara Y., Obata K., Fujimoto N., Yamashita K., Hayakawa T., Shimmei M. Increased levels of stromelysin-1 and tissue inhibitor of metalloproteinases-1 in sera from patients with rheumatoid arthritis. Arthritis Rheum. 1995 Jul;38(7):969–975. doi: 10.1002/art.1780380713. [DOI] [PubMed] [Google Scholar]
- Zafarullah M., Pelletier J. P., Cloutier J. M., Martel-Pelletier J. Elevated metalloproteinase and tissue inhibitor of metalloproteinase mRNA in human osteoarthritic synovia. J Rheumatol. 1993 Apr;20(4):693–697. [PubMed] [Google Scholar]
- Zhang J., Fujimoto N., Iwata K., Sakai T., Okada Y., Hayakawa T. A one-step sandwich enzyme immunoassay for human matrix metalloproteinase 1 (interstitial collagenase) using monoclonal antibodies. Clin Chim Acta. 1993 Oct 15;219(1-2):1–14. doi: 10.1016/0009-8981(93)90192-7. [DOI] [PubMed] [Google Scholar]


