Abstract
OBJECTIVES—Rheumatoid arthritis (RA) is a chronic joint disease associated with certain HLA-DR alleles expressing the QK/RRAA motif or shared epitope. The Epstein-Barr virus (EBV) has been suspected to be a causative factor for RA. The EBV gp110, a glycoprotein of the replicative cycle that contains a copy of the shared epitope, constitutes an important target in the immune control of EBV replication. This study evaluated the specific T cell response to EBV gp110 in patients with RA expressing or not the shared epitope and examined whether this immune cellular response might be related to disease activity and severity. METHODS—25 patients with RA were studied and compared with 25 healthy controls. Disease activity was assessed by biochemical markers of inflammation (erythrocyte sedimentation rate (ESR) and C reactive protein (CRP) levels). Disease severity was defined by extra-articular disease (vasculitis, subcutaneous nodules, or other organ disease). The frequencies of peripheral blood T cells specific for EBV gp110 and a control protein (total protein extract from Escherichia coli) were determined by direct limiting dilution analysis without preliminary bulk culture. RESULTS—The gp110 precursor frequencies ranged from 0 to 20 × 10−6 in patients with RA and controls. The mean gp110 T cell precursor frequency was lower in patients with RA (SD 3.2 (4.4) × 10-6) than in healthy controls (4.1 (3.8) × 10-6) (p = 0.02). No difference was found for the control protein (p = 0.09). Both shared epitope positive and negative patients with RA responded to gp110, without significant difference. A negative correlation between both ESR and CRP levels and the gp110 T cell response was found (r = -0.71, p<0.0001 and r = -0.42, p = 0.038, respectively). Finally, patients with extra-articular disease displayed the lowest immune cellular response to EBV gp110. CONCLUSION—These results suggest that patients with RA have a decreased T cell response to EBV gp110. Since gp110 is an important protein in the control of EBV replication, this might lead to a poor control of EBV infection, chronic exposure to other EBV antigens, and thus to a chronic inflammatory response in patients with RA.
Full Text
The Full Text of this article is available as a PDF (145.0 KB).
Figure 1 .

Correlation between gp110 T cell precursor frequencies and erythrocyte sedimentation rate (ESR) and C reactive protein (CRP) levels. * Spearman rank order test.
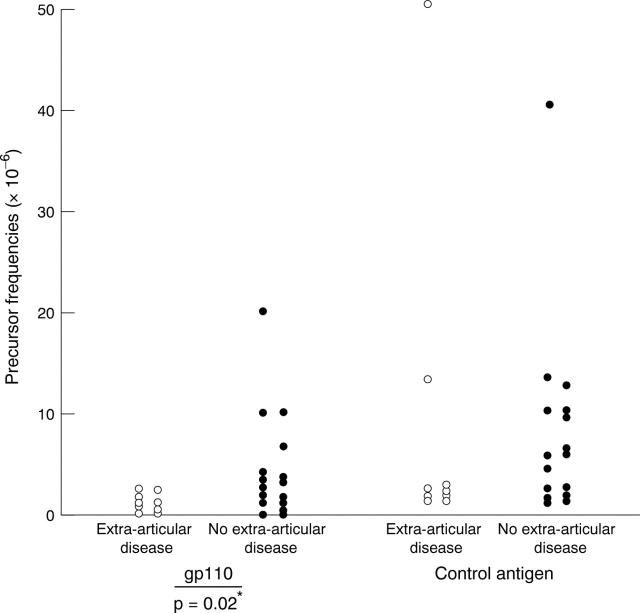
Figure 2 .
GP110 and control antigen T cell precursor frequencies in patients with RA, with and without extra-articular disease. *Fisher's exact test.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albani S., Ravelli A., Massa M., De Benedetti F., Andree G., Roudier J., Martini A., Carson D. A. Immune responses to the Escherichia coli dnaJ heat shock protein in juvenile rheumatoid arthritis and their correlation with disease activity. J Pediatr. 1994 Apr;124(4):561–565. doi: 10.1016/s0022-3476(05)83134-8. [DOI] [PubMed] [Google Scholar]
- Arnett F. C., Edworthy S. M., Bloch D. A., McShane D. J., Fries J. F., Cooper N. S., Healey L. A., Kaplan S. R., Liang M. H., Luthra H. S. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum. 1988 Mar;31(3):315–324. doi: 10.1002/art.1780310302. [DOI] [PubMed] [Google Scholar]
- Bonneville M., Scotet E., Peyrat M. A., Saulquin X., Houssaint E. Epstein-Barr virus and rheumatoid arthritis. Rev Rhum Engl Ed. 1998 Jun;65(6):365–368. [PubMed] [Google Scholar]
- Fox R. I., Luppi M., Pisa P., Kang H. I. Potential role of Epstein-Barr virus in Sjögren's syndrome and rheumatoid arthritis. J Rheumatol Suppl. 1992 Jan;32:18–24. [PubMed] [Google Scholar]
- Gong M., Ooka T., Matsuo T., Kieff E. Epstein-Barr virus glycoprotein homologous to herpes simplex virus gB. J Virol. 1987 Feb;61(2):499–508. doi: 10.1128/jvi.61.2.499-508.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jokinen E. I., Möttönen T. T., Hannonen P. J., Mäkelä M., Arvilommi H. S. Prediction of severe rheumatoid arthritis using Epstein-Barr virus. Br J Rheumatol. 1994 Oct;33(10):917–922. doi: 10.1093/rheumatology/33.10.917. [DOI] [PubMed] [Google Scholar]
- Larsen A. How to apply Larsen score in evaluating radiographs of rheumatoid arthritis in long-term studies. J Rheumatol. 1995 Oct;22(10):1974–1975. [PubMed] [Google Scholar]
- Lotz M., Roudier J. Epstein-Barr virus and rheumatoid arthritis: cellular and molecular aspects. Rheumatol Int. 1989;9(3-5):147–152. doi: 10.1007/BF00271872. [DOI] [PubMed] [Google Scholar]
- Luka J., Chase R. C., Pearson G. R. A sensitive enzyme-linked immunosorbent assay (ELISA) against the major EBV-associated antigens. I. Correlation between ELISA and immunofluorescence titers using purified antigens. J Immunol Methods. 1984 Feb 24;67(1):145–156. doi: 10.1016/0022-1759(84)90093-0. [DOI] [PubMed] [Google Scholar]
- Mousavi-Jazi M., Boström L., Lövmark C., Linde A., Brytting M., Sundqvist V. A. Infrequent detection of cytomegalovirus and Epstein-Barr virus DNA in synovial membrane of patients with rheumatoid arthritis. J Rheumatol. 1998 Apr;25(4):623–628. [PubMed] [Google Scholar]
- Pope R. M., Kniker W. T., Talal N., Dauphinee M. Delayed type hypersensitivity in patients with rheumatoid arthritis. J Rheumatol. 1993 Jan;20(1):17–20. [PubMed] [Google Scholar]
- Roudier J., Albani S., Carson D. A. Immune response to peptides from the third hypervariable region of the beta chain of MHC class II molecules. Implications for the immune response to foreign antigens. Semin Cancer Biol. 1991 Oct;2(5):283–285. [PubMed] [Google Scholar]
- Roudier J., Petersen J., Rhodes G. H., Luka J., Carson D. A. Susceptibility to rheumatoid arthritis maps to a T-cell epitope shared by the HLA-Dw4 DR beta-1 chain and the Epstein-Barr virus glycoprotein gp110. Proc Natl Acad Sci U S A. 1989 Jul;86(13):5104–5108. doi: 10.1073/pnas.86.13.5104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scotet E., David-Ameline J., Peyrat M. A., Moreau-Aubry A., Pinczon D., Lim A., Even J., Semana G., Berthelot J. M., Breathnach R. T cell response to Epstein-Barr virus transactivators in chronic rheumatoid arthritis. J Exp Med. 1996 Nov 1;184(5):1791–1800. doi: 10.1084/jem.184.5.1791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharrock C. E., Kaminski E., Man S. Limiting dilution analysis of human T cells: a useful clinical tool. Immunol Today. 1990 Aug;11(8):281–286. doi: 10.1016/0167-5699(90)90113-n. [DOI] [PubMed] [Google Scholar]
- Tanner J. E., Diaz-Mitoma F., Rooney C. M., Alfieri C. Anti-interleukin-10 antibodies in patients with chronic active Epstein-Barr virus infection. J Infect Dis. 1997 Dec;176(6):1454–1461. doi: 10.1086/514141. [DOI] [PubMed] [Google Scholar]
- Tosato G., Steinberg A. D., Blaese R. M. Defective EBV-specific suppressor T-cell function in rheumatoid arthritis. N Engl J Med. 1981 Nov 19;305(21):1238–1243. doi: 10.1056/NEJM198111193052102. [DOI] [PubMed] [Google Scholar]
- Vaughan J. H. Viruses and autoimmune disease. J Rheumatol. 1996 Nov;23(11):1831–1833. [PubMed] [Google Scholar]
- Vaughan R. W., Lanchbury J. S., Marsh S. G., Hall M. A., Bodmer J. G., Welsh K. I. The application of oligonucleotide probes to HLA class II typing of the DRB sub-region. Tissue Antigens. 1990 Oct;36(4):149–155. doi: 10.1111/j.1399-0039.1990.tb01821.x. [DOI] [PubMed] [Google Scholar]
- Verwilghen J., Vertessen S., Stevens E. A., Dequeker J., Ceuppens J. L. Depressed T-cell reactivity to recall antigens in rheumatoid arthritis. J Clin Immunol. 1990 Mar;10(2):90–98. doi: 10.1007/BF00918190. [DOI] [PubMed] [Google Scholar]
- Winchester R., Dwyer E., Rose S. The genetic basis of rheumatoid arthritis. The shared epitope hypothesis. Rheum Dis Clin North Am. 1992 Nov;18(4):761–783. [PubMed] [Google Scholar]
- Zetterquist H., Olerup O. Identification of the HLA-DRB1*04, -DRB1*07, and -DRB1*09 alleles by PCR amplification with sequence-specific primers (PCR-SSP) in 2 hours. Hum Immunol. 1992 May;34(1):64–74. doi: 10.1016/0198-8859(92)90086-3. [DOI] [PubMed] [Google Scholar]