Full Text
The Full Text of this article is available as a PDF (145.2 KB).
Figure 1 .
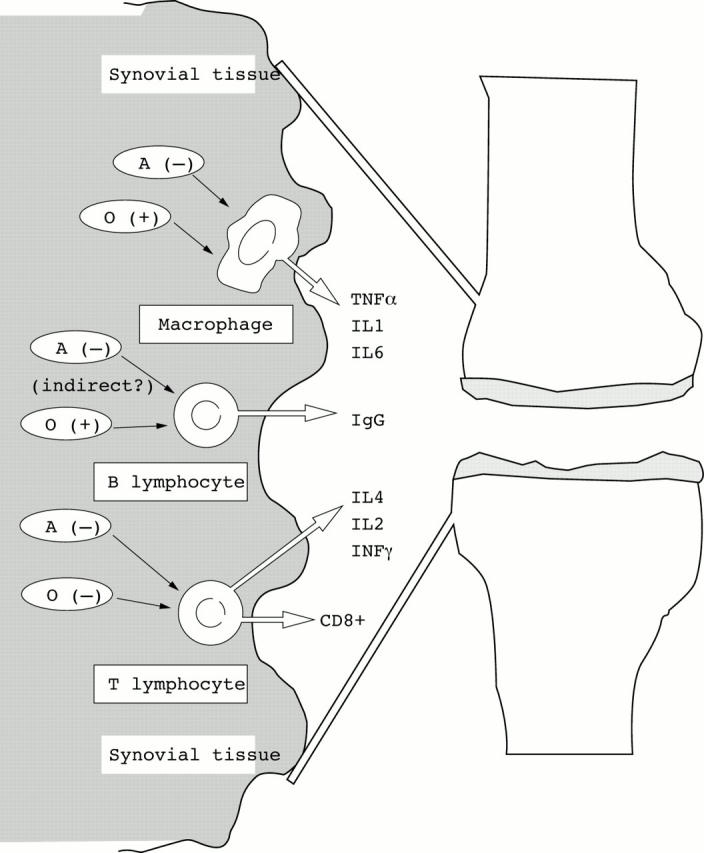
Major stimulatory (+) or inhibitory (−) effects (direct and indirect) of androgens (A) and oestrogens (O) on cytokine/immunoglobulin production by synovial/immune cells at the level of the synovial rheumatoid tissue.
Figure 2 .
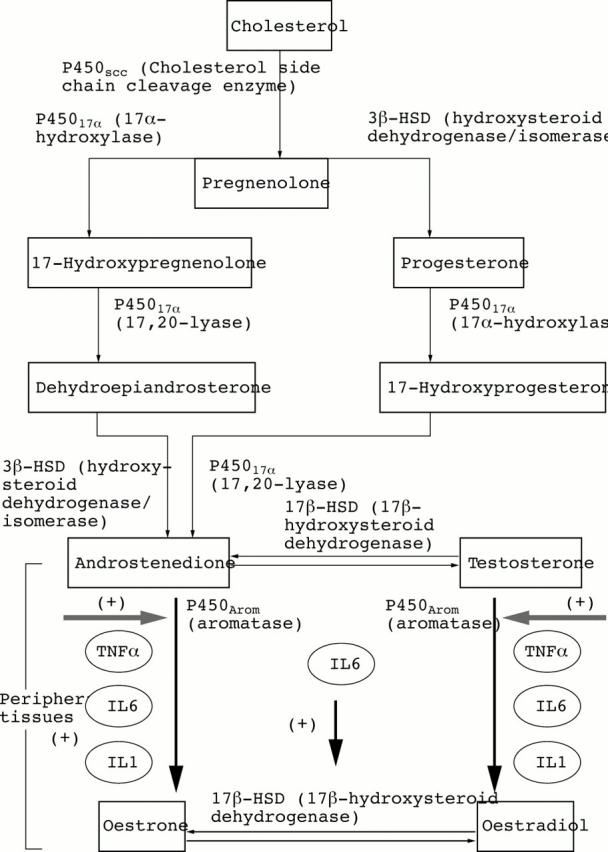
Locally produced inflammatory cytokines (that is, tumour necrosis factor α (TNFα), interleukin 1 (IL1), IL6) can markedly stimulate the aromatase activity (P450Arom) in peripheral tissues (that is, synovial tissue) with subsequent increased conversion of androgens (testosterone and androstenedione) to oestrogens (oestrone and oestradiol, respectively). In addition, IL6 has been found to mediate an increase in reductive 17β-hydroxysteroid dehydrogenase (17β-HSD) activity that converts oestrone to the biologically more active 17β-oestradiol. These effects might explain the altered balance with lower androgens and higher oestrogens found in the synovial RA fluids, as well as their resulting effects on synovial cells.
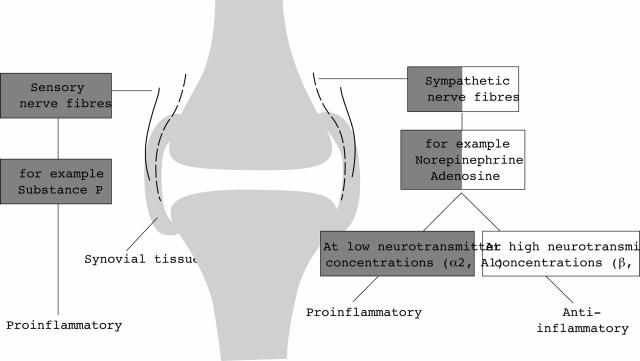
Figure 3 .
Impact of sensory and sympathetic nerve fibres on inflammation in the synovial tissue. The diagram shows the balance of proinflammatory and anti-inflammatory mechanisms. Substance P of sensory nerve fibres and norepinephrine/adenosine at low concentrations seem to be proinflammatory, whereas norepinephrine/adenosine at high concentrations seem to be anti-inflammatory. α2 = alpha 2 adrenergic; β = beta adrenergic; A1 = adenosine receptor 1; A2 = adenosine receptor 2.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abramovici A., Daizade I., Yosipovitch Z., Gibson S. J., Polak J. M. The distribution of peptide-containing nerves in the synovia of the cat knee joint. Histol Histopathol. 1991 Oct;6(4):469–476. [PubMed] [Google Scholar]
- Araneo B. A., Dowell T., Diegel M., Daynes R. A. Dihydrotestosterone exerts a depressive influence on the production of interleukin-4 (IL-4), IL-5, and gamma-interferon, but not IL-2 by activated murine T cells. Blood. 1991 Aug 1;78(3):688–699. [PubMed] [Google Scholar]
- Bijlsma J. W., Cutolo M., Masi A. T., Chikanza I. C. The neuroendocrine immune basis of rheumatic diseases. Immunol Today. 1999 Jul;20(7):298–301. doi: 10.1016/s0167-5699(98)01422-4. [DOI] [PubMed] [Google Scholar]
- Burmester G. R., Stuhlmüller B., Keyszer G., Kinne R. W. Mononuclear phagocytes and rheumatoid synovitis. Mastermind or workhorse in arthritis? Arthritis Rheum. 1997 Jan;40(1):5–18. doi: 10.1002/art.1780400104. [DOI] [PubMed] [Google Scholar]
- Calvo C. F., Chavanel G., Senik A. Substance P enhances IL-2 expression in activated human T cells. J Immunol. 1992 Jun 1;148(11):3498–3504. [PubMed] [Google Scholar]
- Castagnetta L., Cutolo M., Granata O. M., Di Falco M., Bellavia V., Carruba G. Endocrine end-points in rheumatoid arthritis. Ann N Y Acad Sci. 1999 Jun 22;876:180–192. doi: 10.1111/j.1749-6632.1999.tb07637.x. [DOI] [PubMed] [Google Scholar]
- Cerinic M. M., Konttinen Y., Generini S., Cutolo M. Neuropeptides and steroid hormones in arthritis. Curr Opin Rheumatol. 1998 May;10(3):220–235. doi: 10.1097/00002281-199805000-00011. [DOI] [PubMed] [Google Scholar]
- Chrousos G. P. The hypothalamic-pituitary-adrenal axis and immune-mediated inflammation. N Engl J Med. 1995 May 18;332(20):1351–1362. doi: 10.1056/NEJM199505183322008. [DOI] [PubMed] [Google Scholar]
- Cotecchia S., De Blasi A. Glucocorticoids increase beta-adrenoceptors on human intact lymphocytes in vitro. Life Sci. 1984 Dec 3;35(23):2359–2364. doi: 10.1016/0024-3205(84)90528-9. [DOI] [PubMed] [Google Scholar]
- Cutolo M., Accardo S., Villaggio B., Barone A., Sulli A., Balleari E., Bason C., Felli L., Granata O. M., Amodio R. Androgen metabolism and inhibition of interleukin-1 synthesis in primary cultured human synovial macrophages. Mediators Inflamm. 1995;4(2):138–143. doi: 10.1155/S096293519500024X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cutolo M., Accardo S., Villaggio B., Barone A., Sulli A., Coviello D. A., Carabbio C., Felli L., Miceli D., Farruggio R. Androgen and estrogen receptors are present in primary cultures of human synovial macrophages. J Clin Endocrinol Metab. 1996 Feb;81(2):820–827. doi: 10.1210/jcem.81.2.8636310. [DOI] [PubMed] [Google Scholar]
- Cutolo M., Accardo S., Villaggio B., Clerico P., Bagnasco M., Coviello D. A., Carruba G., lo Casto M., Castagnetta L. Presence of estrogen-binding sites on macrophage-like synoviocytes and CD8+, CD29+, CD45RO+ T lymphocytes in normal and rheumatoid synovium. Arthritis Rheum. 1993 Aug;36(8):1087–1097. doi: 10.1002/art.1780360809. [DOI] [PubMed] [Google Scholar]
- Cutolo M. Do sex hormones modulate the synovial macrophages in rheumatoid arthritis? Ann Rheum Dis. 1997 May;56(5):281–284. doi: 10.1136/ard.56.5.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cutolo M. Do sex hormones modulate the synovial macrophages in rheumatoid arthritis? Ann Rheum Dis. 1997 May;56(5):281–284. doi: 10.1136/ard.56.5.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cutolo M. Macrophages as effectors of the immunoendocrinologic interactions in autoimmune rheumatic diseases. Ann N Y Acad Sci. 1999 Jun 22;876:32–42. doi: 10.1111/j.1749-6632.1999.tb07620.x. [DOI] [PubMed] [Google Scholar]
- Cutolo M., Masi A. T. Do androgens influence the pathophysiology of rheumatoid arthritis? Facts and hypotheses. J Rheumatol. 1998 Jun;25(6):1041–1047. [PubMed] [Google Scholar]
- Cutolo M., Prete C., Walker J. Is stress a factor in the pathogenesis of autoimmune rheumatic diseases? Clin Exp Rheumatol. 1999 Sep-Oct;17(5):515–518. [PubMed] [Google Scholar]
- Cutolo M., Sulli A., Barone A., Seriolo B., Accardo S. Macrophages, synovial tissue and rheumatoid arthritis. Clin Exp Rheumatol. 1993 May-Jun;11(3):331–339. [PubMed] [Google Scholar]
- Cutolo M. The role of the hypothalamus-pituitary-adrenocortical and -gonadal axis in rheumatoid arthritis. Clin Exp Rheumatol. 1998 Jan-Feb;16(1):3–6. [PubMed] [Google Scholar]
- Cutolo M., Villaggio B., Barone A., Sulli A., Accardo S., Granata O. M., Castagnetta L. Primary cultures of human synovial macrophages metabolize androgens. Ann N Y Acad Sci. 1996 Apr 30;784:534–541. doi: 10.1111/j.1749-6632.1996.tb16277.x. [DOI] [PubMed] [Google Scholar]
- Cutolo M., Villaggio B., Sulli A., Seriolo B., Giusti M. CYP17 polymorphisms and androgen levels in postmenopausal patients with rheumatoid arthritis. Clin Exp Rheumatol. 2000 May-Jun;18(3):420–421. [PubMed] [Google Scholar]
- Da Silva J. A., Pinto A., Cutolo M., Porto A. Gender differences in adrenal and gonadal responses to inflammatory aggression. Ann N Y Acad Sci. 1999 Jun 22;876:148–151. doi: 10.1111/j.1749-6632.1999.tb07632.x. [DOI] [PubMed] [Google Scholar]
- Dalal M., Kim S., Voskuhl R. R. Testosterone therapy ameliorates experimental autoimmune encephalomyelitis and induces a T helper 2 bias in the autoantigen-specific T lymphocyte response. J Immunol. 1997 Jul 1;159(1):3–6. [PubMed] [Google Scholar]
- DiBattista J. A., Martel-Pelletier J., Cloutier J. M., Pelletier J. P. Modulation of glucocorticoid receptor expression in human articular chondrocytes by cAMP and prostaglandins. J Rheumatol Suppl. 1991 Feb;27:102–105. [PubMed] [Google Scholar]
- Dick W. C., Porter B., Whaley K., Nuki G., Downie W. W., Buchanan W. W. Sympathetic control of synovial blood vessels. Ann Rheum Dis. 1970 Nov;29(6):691–691. doi: 10.1136/ard.29.6.691-b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elenkov I. J., Papanicolaou D. A., Wilder R. L., Chrousos G. P. Modulatory effects of glucocorticoids and catecholamines on human interleukin-12 and interleukin-10 production: clinical implications. Proc Assoc Am Physicians. 1996 Sep;108(5):374–381. [PubMed] [Google Scholar]
- Goldie I., Wellisch M. The presence of nerves in original and regenerated synovial tissue in patients synovectomised for rheumatoid arthritis. Acta Orthop Scand. 1969;40(2):143–152. doi: 10.3109/17453676908989495. [DOI] [PubMed] [Google Scholar]
- Grönblad M., Korkala O., Liesi P., Karaharju E. Innervation of synovial membrane and meniscus. Acta Orthop Scand. 1985 Dec;56(6):484–486. doi: 10.3109/17453678508993040. [DOI] [PubMed] [Google Scholar]
- Heppelmann B., Pawlak M. Sensitisation of articular afferents in normal and inflamed knee joints by substance P in the rat. Neurosci Lett. 1997 Feb 21;223(2):97–100. doi: 10.1016/s0304-3940(97)13408-5. [DOI] [PubMed] [Google Scholar]
- Kanda N., Tamaki K. Estrogen enhances immunoglobulin production by human PBMCs. J Allergy Clin Immunol. 1999 Feb;103(2 Pt 1):282–288. doi: 10.1016/s0091-6749(99)70503-8. [DOI] [PubMed] [Google Scholar]
- Kanda N., Tsuchida T., Tamaki K. Estrogen enhancement of anti-double-stranded DNA antibody and immunoglobulin G production in peripheral blood mononuclear cells from patients with systemic lupus erythematosus. Arthritis Rheum. 1999 Feb;42(2):328–337. doi: 10.1002/1529-0131(199902)42:2<328::AID-ANR16>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- Kanda N., Tsuchida T., Tamaki K. Testosterone inhibits immunoglobulin production by human peripheral blood mononuclear cells. Clin Exp Immunol. 1996 Nov;106(2):410–415. doi: 10.1046/j.1365-2249.1996.d01-842.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanda N., Tsuchida T., Tamaki K. Testosterone suppresses anti-DNA antibody production in peripheral blood mononuclear cells from patients with systemic lupus erythematosus. Arthritis Rheum. 1997 Sep;40(9):1703–1711. doi: 10.1002/art.1780400921. [DOI] [PubMed] [Google Scholar]
- Lam F. Y., Ferrell W. R. Inhibition of carrageenan induced inflammation in the rat knee joint by substance P antagonist. Ann Rheum Dis. 1989 Nov;48(11):928–932. doi: 10.1136/ard.48.11.928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine J. D., Clark R., Devor M., Helms C., Moskowitz M. A., Basbaum A. I. Intraneuronal substance P contributes to the severity of experimental arthritis. Science. 1984 Nov 2;226(4674):547–549. doi: 10.1126/science.6208609. [DOI] [PubMed] [Google Scholar]
- Lieb K., Fiebich B. L., Berger M., Bauer J., Schulze-Osthoff K. The neuropeptide substance P activates transcription factor NF-kappa B and kappa B-dependent gene expression in human astrocytoma cells. J Immunol. 1997 Nov 15;159(10):4952–4958. [PubMed] [Google Scholar]
- Lotz M., Vaughan J. H., Carson D. A. Effect of neuropeptides on production of inflammatory cytokines by human monocytes. Science. 1988 Sep 2;241(4870):1218–1221. doi: 10.1126/science.2457950. [DOI] [PubMed] [Google Scholar]
- Macdiarmid F., Wang D., Duncan L. J., Purohit A., Ghilchick M. W., Reed M. J. Stimulation of aromatase activity in breast fibroblasts by tumor necrosis factor alpha. Mol Cell Endocrinol. 1994 Dec;106(1-2):17–21. doi: 10.1016/0303-7207(94)90181-3. [DOI] [PubMed] [Google Scholar]
- Masi A. T., Bijlsma J. W., Chikanza I. C., Pitzalis C., Cutolo M. Neuroendocrine, immunologic, and microvascular systems interactions in rheumatoid arthritis: physiopathogenetic and therapeutic perspectives. Semin Arthritis Rheum. 1999 Oct;29(2):65–81. doi: 10.1016/s0049-0172(99)80039-0. [DOI] [PubMed] [Google Scholar]
- Masi A. T., Da Silva J. A., Cutolo M. Perturbations of hypothalamic-pituitary-gonadal (HPG) axis and adrenal androgen (AA) functions in rheumatoid arthritis. Baillieres Clin Rheumatol. 1996 May;10(2):295–332. doi: 10.1016/s0950-3579(96)80019-7. [DOI] [PubMed] [Google Scholar]
- Mulherin D., Fitzgerald O., Bresnihan B. Synovial tissue macrophage populations and articular damage in rheumatoid arthritis. Arthritis Rheum. 1996 Jan;39(1):115–124. doi: 10.1002/art.1780390116. [DOI] [PubMed] [Google Scholar]
- Nestler J. E. Interleukin-1 stimulates the aromatase activity of human placental cytotrophoblasts. Endocrinology. 1993 Feb;132(2):566–570. doi: 10.1210/endo.132.2.8425476. [DOI] [PubMed] [Google Scholar]
- Polan M. L., Loukides J., Nelson P., Carding S., Diamond M., Walsh A., Bottomly K. Progesterone and estradiol modulate interleukin-1 beta messenger ribonucleic acid levels in cultured human peripheral monocytes. J Clin Endocrinol Metab. 1989 Dec;69(6):1200–1206. doi: 10.1210/jcem-69-6-1200. [DOI] [PubMed] [Google Scholar]
- Purohit A., Ghilchik M. W., Duncan L., Wang D. Y., Singh A., Walker M. M., Reed M. J. Aromatase activity and interleukin-6 production by normal and malignant breast tissues. J Clin Endocrinol Metab. 1995 Oct;80(10):3052–3058. doi: 10.1210/jcem.80.10.7559896. [DOI] [PubMed] [Google Scholar]
- Renz H., Gong J. H., Schmidt A., Nain M., Gemsa D. Release of tumor necrosis factor-alpha from macrophages. Enhancement and suppression are dose-dependently regulated by prostaglandin E2 and cyclic nucleotides. J Immunol. 1988 Oct 1;141(7):2388–2393. [PubMed] [Google Scholar]
- Riches D. W., Watkins J. L., Henson P. M., Stanworth D. R. Regulation of macrophage lysosomal secretion by adenosine, adenosine phosphate esters, and related structural analogues of adenosine. J Leukoc Biol. 1985 May;37(5):545–557. doi: 10.1002/jlb.37.5.545. [DOI] [PubMed] [Google Scholar]
- Sanders V. M., Baker R. A., Ramer-Quinn D. S., Kasprowicz D. J., Fuchs B. A., Street N. E. Differential expression of the beta2-adrenergic receptor by Th1 and Th2 clones: implications for cytokine production and B cell help. J Immunol. 1997 May 1;158(9):4200–4210. [PubMed] [Google Scholar]
- Schmidt M., Kreutz M., Löffler G., Schölmerich J., Straub R. H. Conversion of dehydroepiandrosterone to downstream steroid hormones in macrophages. J Endocrinol. 2000 Feb;164(2):161–169. doi: 10.1677/joe.0.1640161. [DOI] [PubMed] [Google Scholar]
- Serra M. C., Calzetti F., Ceska M., Cassatella M. A. Effect of substance P on superoxide anion and IL-8 production by human PMNL. Immunology. 1994 May;82(1):63–69. [PMC free article] [PubMed] [Google Scholar]
- Speirs V., Adams E. F., White M. C. The anti-estrogen tamoxifen blocks the stimulatory effects of interleukin-6 on 17 beta-hydroxysteroid dehydrogenase activity in MCF-7 cells. J Steroid Biochem Mol Biol. 1993 Nov;46(5):605–611. doi: 10.1016/0960-0760(93)90188-3. [DOI] [PubMed] [Google Scholar]
- Spengler R. N., Allen R. M., Remick D. G., Strieter R. M., Kunkel S. L. Stimulation of alpha-adrenergic receptor augments the production of macrophage-derived tumor necrosis factor. J Immunol. 1990 Sep 1;145(5):1430–1434. [PubMed] [Google Scholar]
- Spengler R. N., Chensue S. W., Giacherio D. A., Blenk N., Kunkel S. L. Endogenous norepinephrine regulates tumor necrosis factor-alpha production from macrophages in vitro. J Immunol. 1994 Mar 15;152(6):3024–3031. [PubMed] [Google Scholar]
- Sthoeger Z. M., Chiorazzi N., Lahita R. G. Regulation of the immune response by sex hormones. I. In vitro effects of estradiol and testosterone on pokeweed mitogen-induced human B cell differentiation. J Immunol. 1988 Jul 1;141(1):91–98. [PubMed] [Google Scholar]
- Straub R. H., Zeuner M., Antoniou E., Schölmerich J., Lang B. Dehydroepiandrosterone sulfate is positively correlated with soluble interleukin 2 receptor and soluble intercellular adhesion molecule in systemic lupus erythematosus. J Rheumatol. 1996 May;23(5):856–861. [PubMed] [Google Scholar]
- Straub R. H., Zeuner M., Lock G., Schölmerich J., Lang B. High prolactin and low dehydroepiandrosterone sulphate serum levels in patients with severe systemic sclerosis. Br J Rheumatol. 1997 Apr;36(4):426–432. doi: 10.1093/rheumatology/36.4.426. [DOI] [PubMed] [Google Scholar]
- Suenaga R., Evans M. J., Mitamura K., Rider V., Abdou N. I. Peripheral blood T cells and monocytes and B cell lines derived from patients with lupus express estrogen receptor transcripts similar to those of normal cells. J Rheumatol. 1998 Jul;25(7):1305–1312. [PubMed] [Google Scholar]
- Tanabe T., Otani H., Mishima K., Ogawa R., Inagaki C. Mechanisms of oxyradical production in substance P stimulated rheumatoid synovial cells. Rheumatol Int. 1996;16(4):159–167. doi: 10.1007/BF01419729. [DOI] [PubMed] [Google Scholar]
- Ushiyama T., Inoue K., Nishioka J. Expression of estrogen receptor related protein (p29) and estradiol binding in human arthritic synovium. J Rheumatol. 1995 Mar;22(3):421–426. [PubMed] [Google Scholar]
- Walker J. G., Littlejohn G. O., McMurray N. E., Cutolo M. Stress system response and rheumatoid arthritis: a multilevel approach. Rheumatology (Oxford) 1999 Nov;38(11):1050–1057. doi: 10.1093/rheumatology/38.11.1050. [DOI] [PubMed] [Google Scholar]
- Wiedermann C. J., Wiedermann F. J., Apperl A., Kieselbach G., Konwalinka G., Braunsteiner H. In vitro human polymorphonuclear leukocyte chemokinesis and human monocyte chemotaxis are different activities of aminoterminal and carboxyterminal substance P. Naunyn Schmiedebergs Arch Pharmacol. 1989 Aug;340(2):185–190. doi: 10.1007/BF00168967. [DOI] [PubMed] [Google Scholar]
- Wilder R. L. Neuroendocrine-immune system interactions and autoimmunity. Annu Rev Immunol. 1995;13:307–338. doi: 10.1146/annurev.iy.13.040195.001515. [DOI] [PubMed] [Google Scholar]
- Zvaifler N. J. Macrophages and the synovial lining. Scand J Rheumatol Suppl. 1995;101:67–75. doi: 10.3109/03009749509100904. [DOI] [PubMed] [Google Scholar]
- de la Torre B., Hedman M., Nilsson E., Olesen O., Thörner A. Relationship between blood and joint tissue DHEAS levels in rheumatoid arthritis and osteoarthritis. Clin Exp Rheumatol. 1993 Nov-Dec;11(6):597–601. [PubMed] [Google Scholar]
- van Lent P. L., Holthuysen A. E., van den Bersselaar L., van Rooijen N., van de Putte L. B., van den Berg W. B. Role of macrophage-like synovial lining cells in localization and expression of experimental arthritis. Scand J Rheumatol Suppl. 1995;101:83–89. doi: 10.3109/03009749509100906. [DOI] [PubMed] [Google Scholar]