Abstract
OBJECTIVES—Autoimmune diseases are characterised by the production of autoantibodies against various autoantigens. In the past few years data have been published on a possible role of apoptosis in the development of autoimmunity. These include the finding that several autoantigens become modified (for example, by cleavage) during apoptosis, and the observation that these modified antigens are translocated to the cell surface. When the normal clearance of apoptotic cells somehow is disturbed, such modified antigens might become exposed to the immune system. Because acidic ribosomal P (phospho-) proteins targeted by autoantibodies in systemic lupus erythematosus (SLE) are also concentrated at the surface of apoptotic cells, this study aimed at investigating what modifications occur on these antigens during apoptosis. METHODS—Apoptosis in Jurkat cells was induced by Fas ligand (Fas-L), and the fate of autoantigenic P proteins was analysed in both normal and apoptotic total cell extracts. RESULTS—The autoantigenic P proteins were not cleaved but dephosphorylated during Fas-L induced apoptosis. This dephosphorylation was prevented when caspase activity was inhibited. CONCLUSIONS—As has been shown for other autoantigens targeted by autoantibodies in SLE, P proteins also are modified during apoptosis. P1 and P2 are completely dephosphorylated while P0 is partly dephosphorylated. Because the epitope targeted by autoantibodies normally is phosphorylated, it is possible that the apoptotic dephosphorylation of the antigen might be the trigger for the development of the autoimmune response against P proteins.
Full Text
The Full Text of this article is available as a PDF (135.8 KB).
Figure 1 .
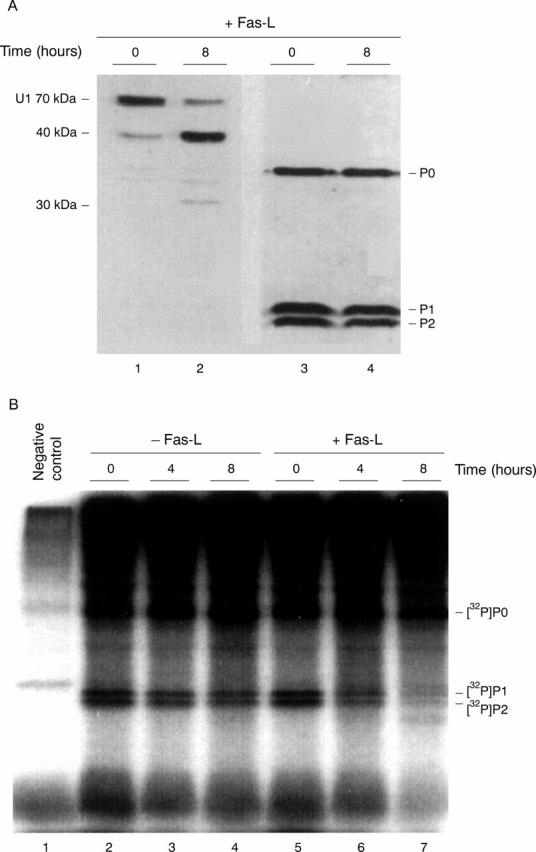
Fas-L induced apoptotic modification of the ribosomal P proteins. (A) Jurkat cells were incubated for 0 and 8 hours with Fas-L. Total protein extracts were analysed by 15% SDS-PAGE and western blotting using anti-P serum (patient 10026) as well as anti-U1 70 kDa patient serum (patient H42). The P proteins (P0, P1, and P2 indicated on the right) are not detectably cleaved (lanes 3, 4). The position of U1 70 kDa (lane 1) and its 40 kDa cleavage product (lane 2) are indicated on the left. The band visible in lane 2 at approximately 30 kDa corresponds to an additional apoptotic cleavage product of the U1 70 kDa protein. (B) 32P labelled Jurkat cells were incubated for 0, 4, and 8 hours with or without Fas-L. 32P labelled P proteins were immunoprecipitated from cell extract with an anti-P serum (lanes 2-7) from a patient with SLE and subjected to autoradiography after 15% SDS-PAGE. Lane 1 represents the negative control (non-specific binding of proteins to protein-A agarose beads).
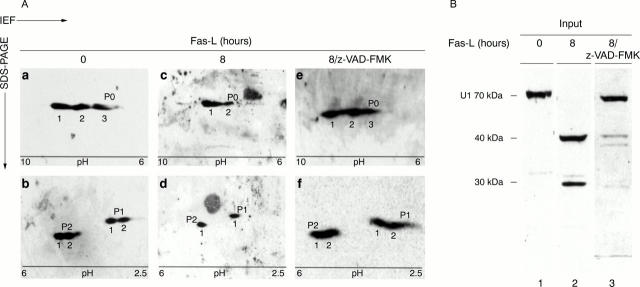
Figure 2 .
Two dimensional IEF/SDS-PAGE analysis of P proteins from normal and apoptotic Jurkat cells. (A) Jurkat cells were incubated for the indicated time period with Fas-L or pretreated with z-VAD-FMK (general caspase inhibitor). Total cell extracts from 2 × 106 cells were fractionated by IEF using a linear gradient of ampholytes (given at the bottom of the figures) followed by 15% SDS-PAGE in the second dimension. Spots of P0, P1, and P2 were detected by immunoblotting using anti-P patient serum 10026. (B) The one dimensional blot was also probed with anti-U1 70 kDa positive patient serum H42 to test the apoptotic status of the cells before the 2D-PAGE analysis (lanes 1, 2, and 3).