Abstract
OBJECTIVE—To investigate whether autoimmunity to YKL-39, a recently cloned cartilage protein, occurs in patients with rheumatoid arthritis (RA). METHODS—Autoantibody to YKL-39 was assayed by enzyme linked immunosorbent assay (ELISA) and western blotting in serum samples from patients with RA, systemic lupus erythematosus (SLE), and healthy donors, using recombinant YKL-39 protein. This reactivity was compared with that against a YKL-39 homologue, YKL-40 (human cartilage gp-39/chondrex), which has been reported to be an autoantigen in RA. RESULTS—Autoantibody to YKL-39 was detected in seven of 87 patients with RA (8%), but not in serum samples from patients with SLE or healthy donors. YKL-40 reactivity was found in only one of 87 RA serum samples (1%), with no cross reactivity to YKL-39. CONCLUSION—The existence of anti-YKL-39 antibody in a subset of patients with RA is reported here for the first time. Further, it was shown that the immune response to YKL-39 was independent of that to YKL-40. Clarification of the antibody and T cell responses to autoantigens derived from chondrocyte, cartilage, or other joint components may lead to a better understanding of the pathophysiology of joint destruction in patients with RA.
Full Text
The Full Text of this article is available as a PDF (162.3 KB).
Figure 1 .
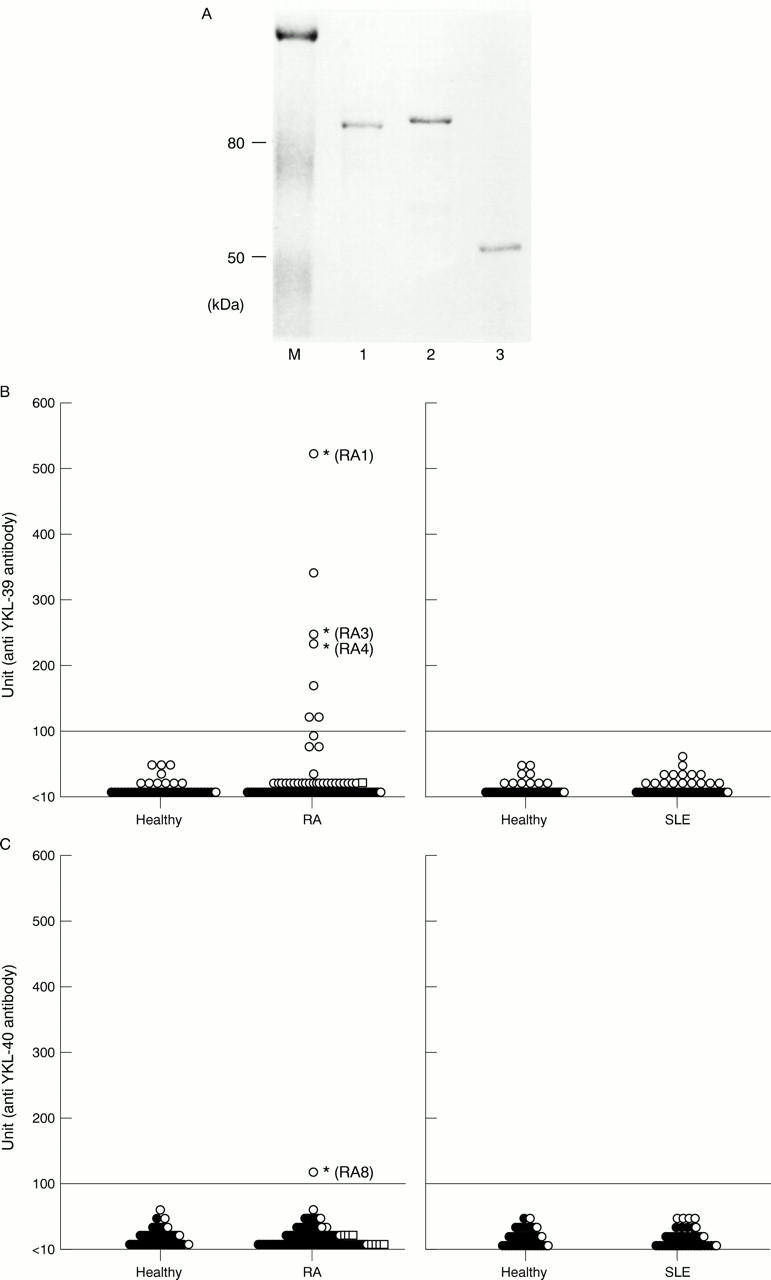
Detection of anti-YKL-39 antibody. (A) Purified fusion proteins, YKL-40 (1), YKL-39 (2), and maltose binding protein (MBP) (3) were electrophoresed on a 10% SDS-PAGE gel and detected by Coomassie brilliant blue staining. (B, C) Detection of autoantibodies to YKL-39 (B) and YKL-40 (C) in serum samples of patients with RA and SLE and healthy control subjects by ELISA. Each symbol indicates a single person. Serum samples from healthy donors matched for age and sex were used as controls. Units of antibodies to YKL-40 in anti-YKL-39 positive sera (open squares in C) and to YKL-39 in anti-YKL-40 positive sera (open circles in B). Data are expressed in arbitrary units as described in "Materials and methods".
Figure 2 .
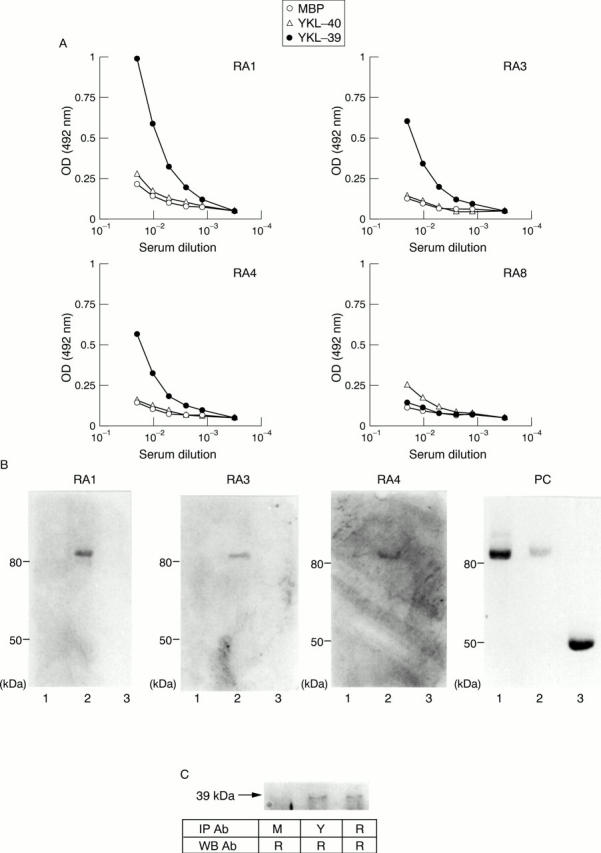
Specificity of autoantibodies to YKL-39 and YKL-40. (A) Antibodies to YKL-39 and YKL-40 were determined by ELISA using serially diluted serum samples. Selected samples are indicated by asterisks in figs 1B and C. Each OD value from maltose binding protein (MBP), YKL-40, and YKL-39 is indicated. (B) YKL-39 positive sera were processed by western blotting to assess reactivity to YKL-40. Recombinant proteins, YKL-40 (1), YKL-39 (2), and MBP (3) were electrophoresed on a 10% SDS-PAGE gel and then western blotted using serum samples from YKL-39 antibody positive patients with RA or anti-MBP antibody as a positive control (PC). (C) Antibodies to YKL-39-MBP fusion protein and those to MBP alone as a negative control were purified from pooled anti-recombinant YKL-39 positive serum samples separately. Then, native YKL-39 in the cell lysate of HCS-2/8 was immunoprecipitated by these purified antibodies and by rabbit anti-YKL-39 polyclonal antibodies as a positive control. The precipitated native YKL-39 was then detected by western blotting using the rabbit anti-YKL-39 antibodies. IP Ab = antibodies used for immunoprecipitation; WB Ab = antibodies used for western blotting; M = antibodies purified by MBP alone; Y = antibodies purified by the recombinant YKL-39 fused with MBP; R = rabbit anti-YKL-39 polyclonal antibodies which were prepared by immunisation of rabbits with the recombinant YKL-39-MBP fusion proteins and subsequent adsorption with MBP.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnett F. C., Edworthy S. M., Bloch D. A., McShane D. J., Fries J. F., Cooper N. S., Healey L. A., Kaplan S. R., Liang M. H., Luthra H. S. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum. 1988 Mar;31(3):315–324. doi: 10.1002/art.1780310302. [DOI] [PubMed] [Google Scholar]
- Bläss S., Specker C., Lakomek H. J., Schneider E. M., Schwochau M. Novel 68 kDa autoantigen detected by rheumatoid arthritis specific antibodies. Ann Rheum Dis. 1995 May;54(5):355–360. doi: 10.1136/ard.54.5.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Cook A. D., Rowley M. J., Mackay I. R., Gough A., Emery P. Antibodies to type II collagen in early rheumatoid arthritis. Correlation with disease progression. Arthritis Rheum. 1996 Oct;39(10):1720–1727. doi: 10.1002/art.1780391015. [DOI] [PubMed] [Google Scholar]
- Hakala B. E., White C., Recklies A. D. Human cartilage gp-39, a major secretory product of articular chondrocytes and synovial cells, is a mammalian member of a chitinase protein family. J Biol Chem. 1993 Dec 5;268(34):25803–25810. [PubMed] [Google Scholar]
- Harvey S., Weisman M., O'Dell J., Scott T., Krusemeier M., Visor J., Swindlehurst C. Chondrex: new marker of joint disease. Clin Chem. 1998 Mar;44(3):509–516. [PubMed] [Google Scholar]
- Hattori T., Fujisawa T., Sasaki K., Yutani Y., Nakanishi T., Takahashi K., Takigawa M. Isolation and characterization of a rheumatoid arthritis-specific antigen (RA-A47) from a human chondrocytic cell line (HCS-2/8). Biochem Biophys Res Commun. 1998 Apr 28;245(3):679–683. doi: 10.1006/bbrc.1998.8505. [DOI] [PubMed] [Google Scholar]
- Hu B., Trinh K., Figueira W. F., Price P. A. Isolation and sequence of a novel human chondrocyte protein related to mammalian members of the chitinase protein family. J Biol Chem. 1996 Aug 9;271(32):19415–19420. doi: 10.1074/jbc.271.32.19415. [DOI] [PubMed] [Google Scholar]
- Johansen J. S., Jensen H. S., Price P. A. A new biochemical marker for joint injury. Analysis of YKL-40 in serum and synovial fluid. Br J Rheumatol. 1993 Nov;32(11):949–955. doi: 10.1093/rheumatology/32.11.949. [DOI] [PubMed] [Google Scholar]
- Matsui T., Kurokawa M., Kobata T., Oki S., Azuma M., Tohma S., Inoue T., Yamamoto K., Nishioka K., Kato T. Autoantibodies to T cell costimulatory molecules in systemic autoimmune diseases. J Immunol. 1999 Apr 1;162(7):4328–4335. [PubMed] [Google Scholar]
- Takigawa M., Tajima K., Pan H. O., Enomoto M., Kinoshita A., Suzuki F., Takano Y., Mori Y. Establishment of a clonal human chondrosarcoma cell line with cartilage phenotypes. Cancer Res. 1989 Jul 15;49(14):3996–4002. [PubMed] [Google Scholar]
- Tan E. M., Cohen A. S., Fries J. F., Masi A. T., McShane D. J., Rothfield N. F., Schaller J. G., Talal N., Winchester R. J. The 1982 revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum. 1982 Nov;25(11):1271–1277. doi: 10.1002/art.1780251101. [DOI] [PubMed] [Google Scholar]
- Verheijden G. F., Rijnders A. W., Bos E., Coenen-de Roo C. J., van Staveren C. J., Miltenburg A. M., Meijerink J. H., Elewaut D., de Keyser F., Veys E. Human cartilage glycoprotein-39 as a candidate autoantigen in rheumatoid arthritis. Arthritis Rheum. 1997 Jun;40(6):1115–1125. doi: 10.1002/art.1780400616. [DOI] [PubMed] [Google Scholar]


