Abstract
OBJECTIVE—Recent studies have demonstrated the short term efficacy of leflunomide. This study evaluates the efficacy and safety of leflunomide and sulfasalazine in rheumatoid arthritis over a two year follow up period. METHODS—358 patients with rheumatoid arthritis in a double blind trial were randomly allocated to receive either leflunomide 20 mg/day, placebo, or sulfasalazine 2 g/day. Those completing six months of treatment (n=230) were given the option to continue in 12 (n=168) and 24 (n=146) month double blinded extensions; the placebo group switched to sulfasalazine. This report compares efficacy and safety of leflunomide with sulfasalazine in the 6, 12, and 24 month patient cohorts. RESULTS—The efficacy seen at six months was maintained at 12 and 24 months. Twenty four month cohorts on leflunomide showed significant improvement compared with sulfasalazine in doctor (−1.46 v −1.11, p=0.03) and patient (−1.61 v −1.04, p<0.001) global assessments, ACR20% response (82% v 60%, p<0.01), and functional ability (Δmean HAQ −0.65 v −0.36, p=0.0149; ΔHAQ disability index −0.89 v −0.60, p=0.059). Improvement in other variables was comparable for the two drugs, including slowing of disease progression. Improved HAQ scores in 6, 12, and 24 month leflunomide cohorts were seen in both non-responders (24%, 29%, 35%, respectively v sulfasalazine 8%, 10%, 27%) and ACR20% responders (leflunomide 63%, 62%, 66% v sulfasalazine 50%, 64%, 44%). Leflunomide is well tolerated at doses of 20 mg. No unexpected adverse events or late toxicity were noted during the two year period. Diarrhoea, nausea, and alopecia were less frequent with continued treatment. CONCLUSION—These long term data confirm that leflunomide is an efficacious and safe disease modifying antirheumatic drug.
Full Text
The Full Text of this article is available as a PDF (238.0 KB).
Figure 1 .

Study design. LEF= leflunomide; SSZ = sulfasalazine; PL = placebo; PL-SSZ = PL group switched to sulfasalazine; R = ACR20% responders; NR = ACR non-responders.
Figure 2 .
Mean changes (SD) in (A) tender and (B) swollen joint counts in 0-6 (LEF = 130, SSZ = 132, PL = 91), 0-12 (LEF = 78, SSZ = 74, PL-SSZ = 37), and 0-24 (LEF = 60, SSZ = 57, PL-SSZ = 25) patient cohorts at 6, 12, and 24 months. LEF = leflunomide; SSZ = sulfasalazine; PL = placebo; PL-SSZ = PL group switched to sulfasalazine. Baseline tender joint counts were: 0-6 month cohort (LEF = 18.8, SSZ = 16.7, PL = 16.3), 0-12 month cohort (LEF = 18.7, SSZ = 15.8, PL = 14.9), and 0-24 month (LEF = 18.4, SSZ = 15.7, PL = 14.1). Baseline swollen joint counts were: 0-6 month cohort (LEF = 16.2, SSZ = 15.3, PL = 15.8), 0-12 month cohort (LEF = 16.3, SSZ = 15.0, PL-SSZ = 14.4), and 0-24 month (LEF = 16.7, SSZ = 15.2, PL-SSZ = 13.9). Numbers in parentheses represent the percentage change from baseline. *p<0.0001 v PL.
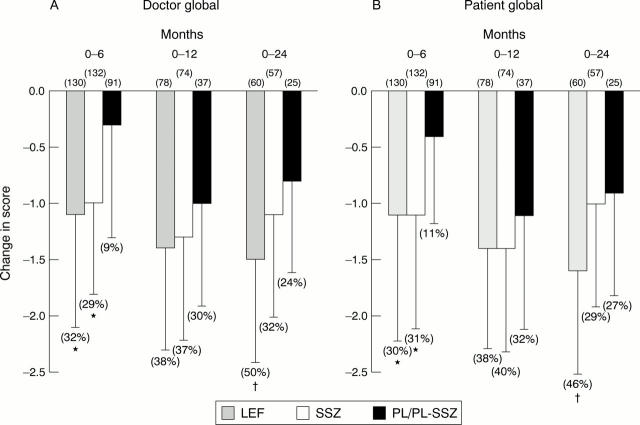
Figure 3 .
Mean changes (SD) in (A) patient and (B) doctor global scores in 0-6 (LEF = 130, SSZ = 132, PL = 91), 0-12 (LEF = 78, SSZ = 74, PL-SSZ= 37), and 0-24 (LEF = 60, SSZ = 57, PL-SSZ = 25) patient cohorts at 6, 12, and 24 months. LEF= leflunomide; SSZ = sulfasalazine; PL = placebo; PL-SSZ = PL group switched to sulfasalazine. Baseline doctor global scores were: 0-6 month cohort (LEF = 3.6, SSZ = 3.5, PL = 3.5), 0-12 month cohort (LEF = 3.7, SSZ = 3.5, PL-SSZ = 3.3), and 0-24 month (LEF = 3.6, SSZ = 3.4, PL-SSZ = 3.3). Baseline patient global scores were: 0-6 month cohort (LEF = 3.7, SSZ = 3.6, PL = 3.6), 0-12 month cohort (LEF = 3.7, SSZ = 3.6, PL-SSZ = 3.4), and 0-24 month (LEF = 3.7, SSZ = 3.5, PL-SSZ = 3.3). Numbers in parentheses represent the percentage improvement from baseline. * p<0.001 v PL; †p<0.001 v SSZ.
Figure 4 .

Changes in Health Assessment Questionnaire (HAQ) scores in 0-6 (LEF = 106, SSZ = 113), 0-12 (LEF = 66, SSZ = 62), and 0-24 (LEF = 51, SSZ = 45) patient cohorts at 6, 12, and 24 months. LEF= leflunomide; SSZ = sulfasalazine. Overall (O) HAQ scores as well as scores in ACR20% responders (R) and non-responders (N) are shown. *p<0.01, v SSZ.
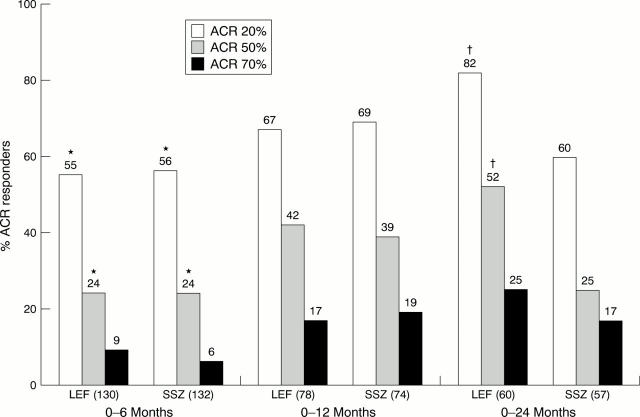
Figure 5 .
ACR20%, 50%, and 70% response rates in 0-6 (LEF = 130, SSZ = 132), 0-12 (LEF = 78, SSZ = 74), and 0-24 (LEF = 60, SSZ = 57) patient cohorts at 6, 12, and 24 months. LEF= leflunomide; SSZ = sulfasalazine. *p<0.05, v SSZ; †p<0.01, v SSZ.
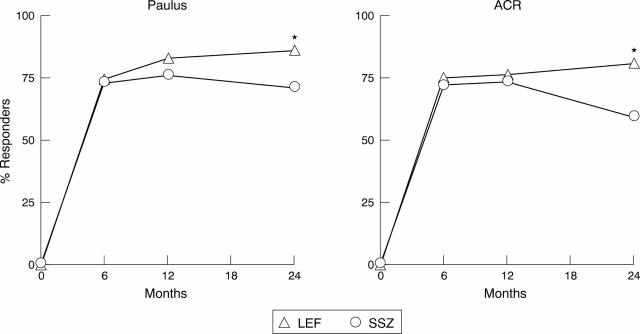
Figure 6 .
Response rates (ACR20% and Paulus 20%) in completers (LEF = 60, SSZ = 57) from 0 to 24 months. LEF= leflunomide; SSZ = sulfasalazine. *p<0.05, v SSZ.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bartlett R. R., Anagnostopulos H., Zielinski T., Mattar T., Schleyerbach R. Effects of leflunomide on immune responses and models of inflammation. Springer Semin Immunopathol. 1993;14(4):381–394. doi: 10.1007/BF00192310. [DOI] [PubMed] [Google Scholar]
- Bax D. E., Amos R. S. Sulphasalazine: a safe, effective agent for prolonged control of rheumatoid arthritis. A comparison with sodium aurothiomalate. Ann Rheum Dis. 1985 Mar;44(3):194–198. doi: 10.1136/ard.44.3.194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borg G., Allander E., Lund B., Berg E., Brodin U., Pettersson H., Trang L. Auranofin improves outcome in early rheumatoid arthritis. Results from a 2-year, double blind placebo controlled study. J Rheumatol. 1988 Dec;15(12):1747–1754. [PubMed] [Google Scholar]
- Box S. A., Pullar T. Sulphasalazine in the treatment of rheumatoid arthritis. Br J Rheumatol. 1997 Mar;36(3):382–386. doi: 10.1093/rheumatology/36.3.382. [DOI] [PubMed] [Google Scholar]
- Breedveld F. C., Dayer J. M. Leflunomide: mode of action in the treatment of rheumatoid arthritis. Ann Rheum Dis. 2000 Nov;59(11):841–849. doi: 10.1136/ard.59.11.841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capell H. A., Konetschnik B., Glass R. C. Anti-inflammatory analgesic drug responders and non-responders: a clinico-pharmacological study of flurbiprofen. Br J Clin Pharmacol. 1977 Oct;4(5):623–624. doi: 10.1111/j.1365-2125.1977.tb00797.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capell H. A., Maiden N., Madhok R., Hampson R., Thomson E. A. Intention-to-treat analysis of 200 patients with rheumatoid arthritis 12 years after random allocation to either sulfasalazine or penicillamine. J Rheumatol. 1998 Oct;25(10):1880–1886. [PubMed] [Google Scholar]
- Capell H. A., Porter D. R., Madhok R., Hunter J. A. Second line (disease modifying) treatment in rheumatoid arthritis: which drug for which patient? Ann Rheum Dis. 1993 Jun;52(6):423–428. doi: 10.1136/ard.52.6.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cush J. J., Lipsky P. E., Postlethwaite A. E., Schrohenloher R. E., Saway A., Koopman W. J. Correlation of serologic indicators of inflammation with effectiveness of nonsteroidal antiinflammatory drug therapy in rheumatoid arthritis. Arthritis Rheum. 1990 Jan;33(1):19–28. doi: 10.1002/art.1780330103. [DOI] [PubMed] [Google Scholar]
- Day R. O. Variability in response to NSAID. Agents Actions Suppl. 1985;17:15–19. doi: 10.1007/978-3-0348-7720-6_1. [DOI] [PubMed] [Google Scholar]
- Devlin J., Gough A., Huissoon A., Perkins P., Holder R., Reece R., Arthur V., Emery P. The acute phase and function in early rheumatoid arthritis. C-reactive protein levels correlate with functional outcome. J Rheumatol. 1997 Jan;24(1):9–13. [PubMed] [Google Scholar]
- Donovan S., Hawley S., MacCarthy J., Scott D. L. Tolerability of enteric-coated sulphasalazine in rheumatoid arthritis: results of a co-operating clinics study. Br J Rheumatol. 1990 Jun;29(3):201–204. doi: 10.1093/rheumatology/29.3.201. [DOI] [PubMed] [Google Scholar]
- Drossaers-Bakker K. W., de Buck M., van Zeben D., Zwinderman A. H., Breedveld F. C., Hazes J. M. Long-term course and outcome of functional capacity in rheumatoid arthritis: the effect of disease activity and radiologic damage over time. Arthritis Rheum. 1999 Sep;42(9):1854–1860. doi: 10.1002/1529-0131(199909)42:9<1854::AID-ANR9>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- Déage V., Burger D., Dayer J. M. Exposure of T lymphocytes to leflunomide but not to dexamethasone favors the production by monocytic cells of interleukin-1 receptor antagonist and the tissue-inhibitor of metalloproteinases-1 over that of interleukin-1beta and metalloproteinases. Eur Cytokine Netw. 1998 Dec;9(4):663–668. [PubMed] [Google Scholar]
- Emery P., Breedveld F. C., Lemmel E. M., Kaltwasser J. P., Dawes P. T., Gömör B., Van Den Bosch F., Nordström D., Bjorneboe O., Dahl R. A comparison of the efficacy and safety of leflunomide and methotrexate for the treatment of rheumatoid arthritis. Rheumatology (Oxford) 2000 Jun;39(6):655–665. doi: 10.1093/rheumatology/39.6.655. [DOI] [PubMed] [Google Scholar]
- Fairbanks L. D., Bofill M., Ruckemann K., Simmonds H. A. Importance of ribonucleotide availability to proliferating T-lymphocytes from healthy humans. Disproportionate expansion of pyrimidine pools and contrasting effects of de novo synthesis inhibitors. J Biol Chem. 1995 Dec 15;270(50):29682–29689. [PubMed] [Google Scholar]
- Felson D. T., Anderson J. J., Meenan R. F. Use of short-term efficacy/toxicity tradeoffs to select second-line drugs in rheumatoid arthritis. A metaanalysis of published clinical trials. Arthritis Rheum. 1992 Oct;35(10):1117–1125. doi: 10.1002/art.1780351003. [DOI] [PubMed] [Google Scholar]
- Fox R. I. Mechanism of action of leflunomide in rheumatoid arthritis. J Rheumatol Suppl. 1998 Jul;53:20–26. [PubMed] [Google Scholar]
- Fries J. F., Spitz P., Kraines R. G., Holman H. R. Measurement of patient outcome in arthritis. Arthritis Rheum. 1980 Feb;23(2):137–145. doi: 10.1002/art.1780230202. [DOI] [PubMed] [Google Scholar]
- Goldsmith C. H., Boers M., Bombardier C., Tugwell P. Criteria for clinically important changes in outcomes: development, scoring and evaluation of rheumatoid arthritis patient and trial profiles. OMERACT Committee. J Rheumatol. 1993 Mar;20(3):561–565. [PubMed] [Google Scholar]
- Hazes J. M., Woolf A. D. The bone and joint decade 2000-2010. J Rheumatol. 2000 Jan;27(1):1–3. [PubMed] [Google Scholar]
- Kraan M. C., Reece R. J., Barg E. C., Smeets T. J., Farnell J., Rosenburg R., Veale D. J., Breedveld F. C., Emery P., Tak P. P. Modulation of inflammation and metalloproteinase expression in synovial tissue by leflunomide and methotrexate in patients with active rheumatoid arthritis. Findings in a prospective, randomized, double-blind, parallel-design clinical trial in thirty-nine patients at two centers. Arthritis Rheum. 2000 Aug;43(8):1820–1830. doi: 10.1002/1529-0131(200008)43:8<1820::AID-ANR18>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- Kraan M. C., de Koster B. M., Elferink J. G., Post W. J., Breedveld F. C., Tak P. P. Inhibition of neutrophil migration soon after initiation of treatment with leflunomide or methotrexate in patients with rheumatoid arthritis: findings in a prospective, randomized, double-blind clinical trial in fifteen patients. Arthritis Rheum. 2000 Jul;43(7):1488–1495. doi: 10.1002/1529-0131(200007)43:7<1488::AID-ANR11>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- Kremer J. M. Methotrexate and leflunomide: biochemical basis for combination therapy in the treatment of rheumatoid arthritis. Semin Arthritis Rheum. 1999 Aug;29(1):14–26. doi: 10.1016/s0049-0172(99)80034-1. [DOI] [PubMed] [Google Scholar]
- Larsen A., Dale K., Eek M. Radiographic evaluation of rheumatoid arthritis and related conditions by standard reference films. Acta Radiol Diagn (Stockh) 1977 Jul;18(4):481–491. doi: 10.1177/028418517701800415. [DOI] [PubMed] [Google Scholar]
- Manna S. K., Aggarwal B. B. Immunosuppressive leflunomide metabolite (A77 1726) blocks TNF-dependent nuclear factor-kappa B activation and gene expression. J Immunol. 1999 Feb 15;162(4):2095–2102. [PubMed] [Google Scholar]
- Mladenovic V., Domljan Z., Rozman B., Jajic I., Mihajlovic D., Dordevic J., Popovic M., Dimitrijevic M., Zivkovic M., Campion G. Safety and effectiveness of leflunomide in the treatment of patients with active rheumatoid arthritis. Results of a randomized, placebo-controlled, phase II study. Arthritis Rheum. 1995 Nov;38(11):1595–1603. doi: 10.1002/art.1780381111. [DOI] [PubMed] [Google Scholar]
- Pullar T., Hunter J. A., Capell H. A. Effect of sulphasalazine on the radiological progression of rheumatoid arthritis. Ann Rheum Dis. 1987 May;46(5):398–402. doi: 10.1136/ard.46.5.398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rückemann K., Fairbanks L. D., Carrey E. A., Hawrylowicz C. M., Richards D. F., Kirschbaum B., Simmonds H. A. Leflunomide inhibits pyrimidine de novo synthesis in mitogen-stimulated T-lymphocytes from healthy humans. J Biol Chem. 1998 Aug 21;273(34):21682–21691. doi: 10.1074/jbc.273.34.21682. [DOI] [PubMed] [Google Scholar]
- Scott D. L., Roden S., Marshall T., Kendall M. J. Variations in responses to non-steroidal anti-inflammatory drugs. Br J Clin Pharmacol. 1982 Nov;14(5):691–694. doi: 10.1111/j.1365-2125.1982.tb04958.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharp J. T., Strand V., Leung H., Hurley F., Loew-Friedrich I. Treatment with leflunomide slows radiographic progression of rheumatoid arthritis: results from three randomized controlled trials of leflunomide in patients with active rheumatoid arthritis. Leflunomide Rheumatoid Arthritis Investigators Group. Arthritis Rheum. 2000 Mar;43(3):495–505. doi: 10.1002/1529-0131(200003)43:3<495::AID-ANR4>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- Situnayake R. D., Grindulis K. A., McConkey B. Long-term treatment of rheumatoid arthritis with sulphasalazine, gold, or penicillamine: a comparison using life-table methods. Ann Rheum Dis. 1987 Mar;46(3):177–183. doi: 10.1136/ard.46.3.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smolen J. S., Kalden J. R., Scott D. L., Rozman B., Kvien T. K., Larsen A., Loew-Friedrich I., Oed C., Rosenburg R. Efficacy and safety of leflunomide compared with placebo and sulphasalazine in active rheumatoid arthritis: a double-blind, randomised, multicentre trial. European Leflunomide Study Group. Lancet. 1999 Jan 23;353(9149):259–266. doi: 10.1016/s0140-6736(98)09403-3. [DOI] [PubMed] [Google Scholar]
- Strand V., Cohen S., Schiff M., Weaver A., Fleischmann R., Cannon G., Fox R., Moreland L., Olsen N., Furst D. Treatment of active rheumatoid arthritis with leflunomide compared with placebo and methotrexate. Leflunomide Rheumatoid Arthritis Investigators Group. Arch Intern Med. 1999 Nov 22;159(21):2542–2550. doi: 10.1001/archinte.159.21.2542. [DOI] [PubMed] [Google Scholar]
- Tomlin G. S., Holm M. B., Rogers J. C., Kwoh C. K. Comparison of standard and alternative health assessment questionnaire scoring procedures for documenting functional outcomes in patients with rheumatoid arthritis. J Rheumatol. 1996 Sep;23(9):1524–1530. [PubMed] [Google Scholar]
- Tugwell P., Wells G., Strand V., Maetzel A., Bombardier C., Crawford B., Dorrier C., Thompson A. Clinical improvement as reflected in measures of function and health-related quality of life following treatment with leflunomide compared with methotrexate in patients with rheumatoid arthritis: sensitivity and relative efficiency to detect a treatment effect in a twelve-month, placebo-controlled trial. Leflunomide Rheumatoid Arthritis Investigators Group. Arthritis Rheum. 2000 Mar;43(3):506–514. doi: 10.1002/1529-0131(200003)43:3<506::AID-ANR5>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- Walker J. S., Sheather-Reid R. B., Carmody J. J., Vial J. H., Day R. O. Nonsteroidal antiinflammatory drugs in rheumatoid arthritis and osteoarthritis: support for the concept of "responders" and "nonresponders". Arthritis Rheum. 1997 Nov;40(11):1944–1954. doi: 10.1002/art.1780401105. [DOI] [PubMed] [Google Scholar]
- Weinblatt M. E., Reda D., Henderson W., Giobbie-Hurder A., Williams D., Diani A., Docsa S. Sulfasalazine treatment for rheumatoid arthritis: a metaanalysis of 15 randomized trials. J Rheumatol. 1999 Oct;26(10):2123–2130. [PubMed] [Google Scholar]
- Wells G. A., Tugwell P., Kraag G. R., Baker P. R., Groh J., Redelmeier D. A. Minimum important difference between patients with rheumatoid arthritis: the patient's perspective. J Rheumatol. 1993 Mar;20(3):557–560. [PubMed] [Google Scholar]
- Wolfe F. Adverse drug reactions of DMARDs and DC-ARTs in rheumatoid arthritis. Clin Exp Rheumatol. 1997 May-Jun;15 (Suppl 17):S75–S81. [PubMed] [Google Scholar]
- van Leeuwen M. A., van der Heijde D. M., van Rijswijk M. H., Houtman P. M., van Riel P. L., van de Putte L. B., Limburg P. C. Interrelationship of outcome measures and process variables in early rheumatoid arthritis. A comparison of radiologic damage, physical disability, joint counts, and acute phase reactants. J Rheumatol. 1994 Mar;21(3):425–429. [PubMed] [Google Scholar]
- van de Putte L. B., van Riel P. L. Currently used second-line agents: do they control the disease course? Clin Exp Rheumatol. 1997 May-Jun;15 (Suppl 17):S71–S74. [PubMed] [Google Scholar]
- van der Heijde D. M., van Riel P. L., Nuver-Zwart I. H., Gribnau F. W., vad de Putte L. B. Effects of hydroxychloroquine and sulphasalazine on progression of joint damage in rheumatoid arthritis. Lancet. 1989 May 13;1(8646):1036–1038. doi: 10.1016/s0140-6736(89)92442-2. [DOI] [PubMed] [Google Scholar]