Abstract
BACKGROUND—The occurrence of B cell non-Hodgkin's lymphoma is a complication of Sjögren's syndrome (SS) and, at least in some countries, of chronic hepatitis C virus (HCV) infection. Lymphomas occurring in both diseases share a number of characteristics: predominance of low grade, marginal zone histological type, frequency of mucosal localisation, possible transformation into a large B cell lymphoma, association with asymptomatic low level cryoglobulinaemia, absence of virus within lymphoma cells, but localisation of lymphomas in organs where the chronic viral infection is active in patients with HCV and where the autoimmune disease is active in patients with SS. HYPOTHESIS—It is proposed that in both diseases the first event of lymphomagenesis is the chronic stimulation at the site of the disease of polyclonal B cells secreting rheumatoid factor (RF). Then, that these RF B cells may become monoclonal and disseminate in other organs. The monoclonal secreted RF complexed with polyclonal IgG may cryoprecipitate. The following step would be a chromosomal abnormality (for example, trisomy 3 or bcl-2 translocation) which would confer to these cells a low grade B cell lymphoma comportment. A last event (for example, a mutation of p53) might transform this low grade B cell lymphoma into a high grade, large B cell lymphoma. The non-random utilisation of VH and VL by SS associated lymphoma B cells and the recent demonstration that these lymphoma B cells may display RF activity support the hypothesis that these lymphomas grow through an autoantigen driven process. CONCLUSION—The best preventive treatment of lymphoproliferations occurring in SS probably consists in decreasing the hyperactivation of autoreactive B cells when it is present, allowing the use of immunosuppressive drugs such as methotrexate or even tumour necrosis factor α antagonists, which in theory could favour other types of lymphoproliferation.
Full Text
The Full Text of this article is available as a PDF (92.4 KB).
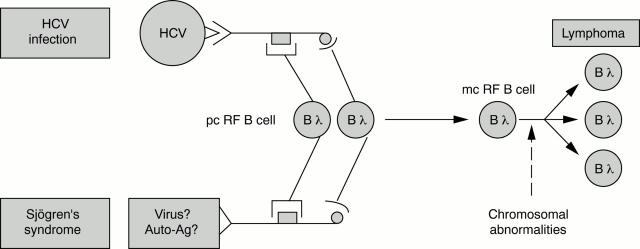
Figure 1 .
Pathogenic hypothesis suggested owing to shared characteristics between lymphomas complicating HCV infection and Sjögren's syndrome. HCV = hepatitis C virus; Bλ = B lymphocyte; pc = polyclonal; mc = monoclonal; RF = rheumatoid factor.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Agnello V., Chung R. T., Kaplan L. M. A role for hepatitis C virus infection in type II cryoglobulinemia. N Engl J Med. 1992 Nov 19;327(21):1490–1495. doi: 10.1056/NEJM199211193272104. [DOI] [PubMed] [Google Scholar]
- Bahler D. W., Miklos J. A., Swerdlow S. H. Ongoing Ig gene hypermutation in salivary gland mucosa-associated lymphoid tissue-type lymphomas. Blood. 1997 May 1;89(9):3335–3344. [PubMed] [Google Scholar]
- Bahler D. W., Swerdlow S. H. Clonal salivary gland infiltrates associated with myoepithelial sialadenitis (Sjögren's syndrome) begin as nonmalignant antigen-selected expansions. Blood. 1998 Mar 15;91(6):1864–1872. [PubMed] [Google Scholar]
- Brezinschek H. P., Foster S. J., Brezinschek R. I., Dörner T., Domiati-Saad R., Lipsky P. E. Analysis of the human VH gene repertoire. Differential effects of selection and somatic hypermutation on human peripheral CD5(+)/IgM+ and CD5(-)/IgM+ B cells. J Clin Invest. 1997 May 15;99(10):2488–2501. doi: 10.1172/JCI119433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan J. K., Ng C. S., Isaacson P. G. Relationship between high-grade lymphoma and low-grade B-cell mucosa-associated lymphoid tissue lymphoma (MALToma) of the stomach. Am J Pathol. 1990 May;136(5):1153–1164. [PMC free article] [PubMed] [Google Scholar]
- De Vita S., Boiocchi M., Sorrentino D., Carbone A., Avellini C., Dolcetti R., Marzotto A., Gloghini A., Bartoli E., Beltrami C. A. Characterization of prelymphomatous stages of B cell lymphoproliferation in Sjögren's syndrome. Arthritis Rheum. 1997 Feb;40(2):318–331. doi: 10.1002/art.1780400217. [DOI] [PubMed] [Google Scholar]
- De Vita S., Sacco C., Sansonno D., Gloghini A., Dammacco F., Crovatto M., Santini G., Dolcetti R., Boiocchi M., Carbone A. Characterization of overt B-cell lymphomas in patients with hepatitis C virus infection. Blood. 1997 Jul 15;90(2):776–782. [PubMed] [Google Scholar]
- Dierlamm J., Pittaluga S., Wlodarska I., Stul M., Thomas J., Boogaerts M., Michaux L., Driessen A., Mecucci C., Cassiman J. J. Marginal zone B-cell lymphomas of different sites share similar cytogenetic and morphologic features. Blood. 1996 Jan 1;87(1):299–307. [PubMed] [Google Scholar]
- Du M., Peng H., Singh N., Isaacson P. G., Pan L. The accumulation of p53 abnormalities is associated with progression of mucosa-associated lymphoid tissue lymphoma. Blood. 1995 Dec 15;86(12):4587–4593. [PubMed] [Google Scholar]
- Ferri C., Caracciolo F., Zignego A. L., La Civita L., Monti M., Longombardo G., Lombardini F., Greco F., Capochiani E., Mazzoni A. Hepatitis C virus infection in patients with non-Hodgkin's lymphoma. Br J Haematol. 1994 Oct;88(2):392–394. doi: 10.1111/j.1365-2141.1994.tb05036.x. [DOI] [PubMed] [Google Scholar]
- Fox R. I., Howell F. V., Bone R. C., Michelson P. Primary Sjogren syndrome: clinical and immunopathologic features. Semin Arthritis Rheum. 1984 Nov;14(2):77–105. doi: 10.1016/0049-0172(84)90001-5. [DOI] [PubMed] [Google Scholar]
- Ihrler S., Baretton G. B., Menauer F., Blasenbreu-Vogt S., Löhrs U. Sjögren's syndrome and MALT lymphomas of salivary glands: a DNA-cytometric and interphase-cytogenetic study. Mod Pathol. 2000 Jan;13(1):4–12. doi: 10.1038/modpathol.3880002. [DOI] [PubMed] [Google Scholar]
- Ivanovski M., Silvestri F., Pozzato G., Anand S., Mazzaro C., Burrone O. R., Efremov D. G. Somatic hypermutation, clonal diversity, and preferential expression of the VH 51p1/VL kv325 immunoglobulin gene combination in hepatitis C virus-associated immunocytomas. Blood. 1998 Apr 1;91(7):2433–2442. [PubMed] [Google Scholar]
- Jordan R., Diss T. C., Lench N. J., Isaacson P. G., Speight P. M. Immunoglobulin gene rearrangements in lymphoplasmacytic infiltrates of labial salivary glands in Sjögren's syndrome. A possible predictor of lymphoma development. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1995 Jun;79(6):723–729. doi: 10.1016/s1079-2104(05)80307-5. [DOI] [PubMed] [Google Scholar]
- Kassan S. S., Thomas T. L., Moutsopoulos H. M., Hoover R., Kimberly R. P., Budman D. R., Costa J., Decker J. L., Chused T. M. Increased risk of lymphoma in sicca syndrome. Ann Intern Med. 1978 Dec;89(6):888–892. doi: 10.7326/0003-4819-89-6-888. [DOI] [PubMed] [Google Scholar]
- Kitay-Cohen Y., Amiel A., Hilzenrat N., Buskila D., Ashur Y., Fejgin M., Gaber E., Safadi R., Tur-Kaspa R., Lishner M. Bcl-2 rearrangement in patients with chronic hepatitis C associated with essential mixed cryoglobulinemia type II. Blood. 2000 Oct 15;96(8):2910–2912. [PubMed] [Google Scholar]
- Kruize A. A., Hené R. J., van der Heide A., Bodeutsch C., de Wilde P. C., van Bijsterveld O. P., de Jong J., Feltkamp T. E., Kater L., Bijlsma J. W. Long-term followup of patients with Sjögren's syndrome. Arthritis Rheum. 1996 Feb;39(2):297–303. doi: 10.1002/art.1780390219. [DOI] [PubMed] [Google Scholar]
- Mariette X., Agbalika F., Daniel M. T., Bisson M., Lagrange P., Brouet J. C., Morinet F. Detection of human T lymphotropic virus type I tax gene in salivary gland epithelium from two patients with Sjögren's syndrome. Arthritis Rheum. 1993 Oct;36(10):1423–1428. doi: 10.1002/art.1780361015. [DOI] [PubMed] [Google Scholar]
- Mariette X., Gozlan J., Clerc D., Bisson M., Morinet F. Detection of Epstein-Barr virus DNA by in situ hybridization and polymerase chain reaction in salivary gland biopsy specimens from patients with Sjögren's syndrome. Am J Med. 1991 Mar;90(3):286–294. [PubMed] [Google Scholar]
- Mariette X., Sibilia J., Delaforge C., Bengoufa D., Brouet J. C., Soussi T. Anti-p53 antibodies are rarely detected in serum of patients with rheumatoid arthritis and Sjögren's syndrome. J Rheumatol. 1999 Aug;26(8):1672–1675. [PubMed] [Google Scholar]
- Martin T., Weber J. C., Levallois H., Labouret N., Soley A., Koenig S., Korganow A. S., Pasquali J. L. Salivary gland lymphomas in patients with Sjögren's syndrome may frequently develop from rheumatoid factor B cells. Arthritis Rheum. 2000 Apr;43(4):908–916. doi: 10.1002/1529-0131(200004)43:4<908::AID-ANR24>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- Miklos J. A., Swerdlow S. H., Bahler D. W. Salivary gland mucosa-associated lymphoid tissue lymphoma immunoglobulin V(H) genes show frequent use of V1-69 with distinctive CDR3 features. Blood. 2000 Jun 15;95(12):3878–3884. [PubMed] [Google Scholar]
- Nagai K. [Sjögren's syndrome associated with non-Hodgkin's lymphoma]. Nihon Rinsho. 1995 Oct;53(10):2574–2579. [PubMed] [Google Scholar]
- Nizze H., Cogliatti S. B., von Schilling C., Feller A. C., Lennert K. Monocytoid B-cell lymphoma: morphological variants and relationship to low-grade B-cell lymphoma of the mucosa-associated lymphoid tissue. Histopathology. 1991 May;18(5):403–414. doi: 10.1111/j.1365-2559.1991.tb00870.x. [DOI] [PubMed] [Google Scholar]
- Pablos J. L., Carreira P. E., Morillas L., Montalvo G., Ballestin C., Gomez-Reino J. J. Clonally expanded lymphocytes in the minor salivary glands of Sjögren's syndrome patients without lymphoproliferative disease. Arthritis Rheum. 1994 Oct;37(10):1441–1444. doi: 10.1002/art.1780371006. [DOI] [PubMed] [Google Scholar]
- Pariente D., Anaya J. M., Combe B., Jorgensen C., Emberger J. M., Rossi J. F., Sany J. Non-Hodgkin's lymphoma associated with primary Sjögren's syndrome. Eur J Med. 1992 Oct;1(6):337–342. [PubMed] [Google Scholar]
- Pawlotsky J. M., Roudot-Thoraval F., Simmonds P., Mellor J., Ben Yahia M. B., André C., Voisin M. C., Intrator L., Zafrani E. S., Duval J. Extrahepatic immunologic manifestations in chronic hepatitis C and hepatitis C virus serotypes. Ann Intern Med. 1995 Feb 1;122(3):169–173. doi: 10.7326/0003-4819-122-3-199502010-00002. [DOI] [PubMed] [Google Scholar]
- Pisa E. K., Pisa P., Kang H. I., Fox R. I. High frequency of t(14;18) translocation in salivary gland lymphomas from Sjögren's syndrome patients. J Exp Med. 1991 Nov 1;174(5):1245–1250. doi: 10.1084/jem.174.5.1245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Royer B., Cazals-Hatem D., Sibilia J., Agbalika F., Cayuela J. M., Soussi T., Maloisel F., Clauvel J. P., Brouet J. C., Mariette X. Lymphomas in patients with Sjogren's syndrome are marginal zone B-cell neoplasms, arise in diverse extranodal and nodal sites, and are not associated with viruses. Blood. 1997 Jul 15;90(2):766–775. [PubMed] [Google Scholar]
- Semsei I., Zeher M., Takács I., Urbán L., Szegedi G., Bachmann M. High frequency of t(14;18) translocation in Sjögren's syndrome: comment on the article by Gellrich et al. Arthritis Rheum. 2000 Apr;43(4):951–952. doi: 10.1002/1529-0131(200004)43:4<951::aid-anr38>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- Skopouli F. N., Dafni U., Ioannidis J. P., Moutsopoulos H. M. Clinical evolution, and morbidity and mortality of primary Sjögren's syndrome. Semin Arthritis Rheum. 2000 Apr;29(5):296–304. doi: 10.1016/s0049-0172(00)80016-5. [DOI] [PubMed] [Google Scholar]
- Sutcliffe N., Inanc M., Speight P., Isenberg D. Predictors of lymphoma development in primary Sjögren's syndrome. Semin Arthritis Rheum. 1998 Oct;28(2):80–87. doi: 10.1016/s0049-0172(98)80040-1. [DOI] [PubMed] [Google Scholar]
- Tapinos N. I., Polihronis M., Moutsopoulos H. M. Lymphoma development in Sjögren's syndrome: novel p53 mutations. Arthritis Rheum. 1999 Jul;42(7):1466–1472. doi: 10.1002/1529-0131(199907)42:7<1466::AID-ANR21>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- Tzioufas A. G., Boumba D. S., Skopouli F. N., Moutsopoulos H. M. Mixed monoclonal cryoglobulinemia and monoclonal rheumatoid factor cross-reactive idiotypes as predictive factors for the development of lymphoma in primary Sjögren's syndrome. Arthritis Rheum. 1996 May;39(5):767–772. doi: 10.1002/art.1780390508. [DOI] [PubMed] [Google Scholar]
- Voulgarelis M., Dafni U. G., Isenberg D. A., Moutsopoulos H. M. Malignant lymphoma in primary Sjögren's syndrome: a multicenter, retrospective, clinical study by the European Concerted Action on Sjögren's Syndrome. Arthritis Rheum. 1999 Aug;42(8):1765–1772. doi: 10.1002/1529-0131(199908)42:8<1765::AID-ANR28>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- Zuckerman E., Zuckerman T., Levine A. M., Douer D., Gutekunst K., Mizokami M., Qian D. G., Velankar M., Nathwani B. N., Fong T. L. Hepatitis C virus infection in patients with B-cell non-Hodgkin lymphoma. Ann Intern Med. 1997 Sep 15;127(6):423–428. doi: 10.7326/0003-4819-127-6-199709150-00002. [DOI] [PubMed] [Google Scholar]
- Zuckerman E., Zuckerman T., Sahar D., Streichman S., Attias D., Sabo E., Yeshurun D., Rowe J. M. The effect of antiviral therapy on t(14;18) translocation and immunoglobulin gene rearrangement in patients with chronic hepatitis C virus infection. Blood. 2001 Mar 15;97(6):1555–1559. doi: 10.1182/blood.v97.6.1555. [DOI] [PubMed] [Google Scholar]
- Zulman J., Jaffe R., Talal N. Evidence that the malignant lymphoma of Sjögren's syndrome is a monoclonal B-cell neoplasm. N Engl J Med. 1978 Nov 30;299(22):1215–1220. doi: 10.1056/NEJM197811302992204. [DOI] [PubMed] [Google Scholar]