Abstract
OBJECTIVE—To provide data on (a) the probability of detecting antinuclear antibodies (ANA) in a large and consecutive cohort of serum samples referred for ANA testing and (b) the probability of detecting more specific antinuclear reactivities (anti-DNA and anti-extractable nuclear antigens (anti-ENA)) in serum samples with a positive screening test (indirect immunofluorescence on HEp-2 cells). METHODS—Serum samples from 10 550 consecutive patients sent to the laboratory for ANA detection were analysed. In ANA positive serum samples (23.5% of referred serum samples), ANA were identified by indirect immunofluorescence on Crithidia, by immunodiffusion, and by line immunoassay. Because anti-SSA antibodies were the most frequently identified ANA, sensitively detected by line immunoassay, additional immunoassays were developed to confirm the specificity of the line immunoassay result. RESULTS—At least one fine reactivity could be identified in 21.1% of ANA positive serum samples: anti-dsDNA in 3.2%; anti-ENA (anti-SSA 10.5%, anti-SSB 6.7%, anti-RNP 2.7%, anti-Sm 1.8%, anti-Scl70 1.2%, anti-Jo-1 0.2%) in 15.8%, rRNP and anti-Cenp-B in respectively 0.5% and 4.0%. Multiple reactivities were found in 7.9%. For anti-ENA antibodies, line immunoassay was more sensitive than immunodiffusion (15.4% v 7.7%; p<0.0001). The sensitive detection of anti-SSA antibodies by line immunoassay was confirmed by additional assays. CONCLUSIONS—The data from this analysis are useful in estimating the probabilities of detecting specific ANA. Line immunoassay was shown to be a sensitive test for the detection of anti-ENA antibodies.
Full Text
The Full Text of this article is available as a PDF (176.7 KB).
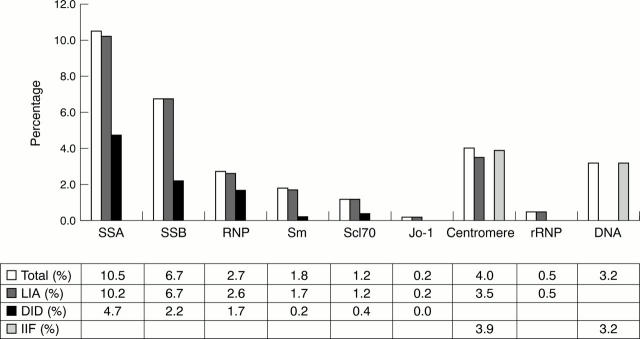
Figure 1 .
Identification of fine reactivities in ANA positive serum samples. LIA = line immunoassay; DID = double immunodiffusion; IIF = indirect immunofluorescence on HEp-2 cells (for anticentromere pattern) or Crithidia luciliae (for anti-dsDNA).
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Buyon J. P., Slade S. G., Chan E. K., Tan E. M., Winchester R. Effective separation of the 52 kDa SSA/Ro polypeptide from the 48 kDa SSB/La polypeptide by altering conditions of polyacrylamide gel electrophoresis. J Immunol Methods. 1990 May 25;129(2):207–210. doi: 10.1016/0022-1759(90)90440-7. [DOI] [PubMed] [Google Scholar]
- Emlen W., O'Neill L. Clinical significance of antinuclear antibodies: comparison of detection with immunofluorescence and enzyme-linked immunosorbent assays. Arthritis Rheum. 1997 Sep;40(9):1612–1618. doi: 10.1002/art.1780400910. [DOI] [PubMed] [Google Scholar]
- Frank M. B., McCubbin V., Trieu E., Wu Y., Isenberg D. A., Targoff I. N. The association of anti-Ro52 autoantibodies with myositis and scleroderma autoantibodies. J Autoimmun. 1999 Mar;12(2):137–142. doi: 10.1006/jaut.1998.0265. [DOI] [PubMed] [Google Scholar]
- Fritzler M. J., Miller B. J. Detection of autoantibodies to SS-A/Ro by indirect immunofluorescence using a transfected and overexpressed human 60 kD Ro autoantigen in HEp-2 cells. J Clin Lab Anal. 1995;9(3):218–224. doi: 10.1002/jcla.1860090312. [DOI] [PubMed] [Google Scholar]
- Homburger H. A. Cascade testing for autoantibodies in connective tissue diseases. Mayo Clin Proc. 1995 Feb;70(2):183–184. doi: 10.4065/70.2.183. [DOI] [PubMed] [Google Scholar]
- Kavanaugh A., Tomar R., Reveille J., Solomon D. H., Homburger H. A. Guidelines for clinical use of the antinuclear antibody test and tests for specific autoantibodies to nuclear antigens. American College of Pathologists. Arch Pathol Lab Med. 2000 Jan;124(1):71–81. doi: 10.5858/2000-124-0071-GFCUOT. [DOI] [PubMed] [Google Scholar]
- Keech C. L., McCluskey J., Gordon T. P. Transfection and overexpression of the human 60-kDa Ro/SS-A autoantigen in HEp-2 cells. Clin Immunol Immunopathol. 1994 Oct;73(1):146–151. doi: 10.1006/clin.1994.1181. [DOI] [PubMed] [Google Scholar]
- Meheus L., van Venrooij W. J., Wiik A., Charles P. J., Tzioufas A. G., Meyer O., Steiner G., Gianola D., Bombardieri S., Union A. Multicenter validation of recombinant, natural and synthetic antigens used in a single multiparameter assay for the detection of specific anti-nuclear autoantibodies in connective tissue disorders. Clin Exp Rheumatol. 1999 Mar-Apr;17(2):205–214. [PubMed] [Google Scholar]
- Peene I., Van Ael W., Vandenbossche M., Vervaet T., Veys E., De Keyser F. Sensitivity of the HEp-2000 substrate for the detection of anti-SSA/Ro60 antibodies. Clin Rheumatol. 2000;19(4):291–295. doi: 10.1007/s100670070048. [DOI] [PubMed] [Google Scholar]
- Pourmand N., Pettersson I. The Zn2+ binding domain of the human Ro 52 kDa protein is a target for conformation-dependent autoantibodies. J Autoimmun. 1998 Feb;11(1):11–17. doi: 10.1006/jaut.1997.0171. [DOI] [PubMed] [Google Scholar]
- Slater C. A., Davis R. B., Shmerling R. H. Antinuclear antibody testing. A study of clinical utility. Arch Intern Med. 1996 Jul 8;156(13):1421–1425. [PubMed] [Google Scholar]
- Tan E. M., Feltkamp T. E., Smolen J. S., Butcher B., Dawkins R., Fritzler M. J., Gordon T., Hardin J. A., Kalden J. R., Lahita R. G. Range of antinuclear antibodies in "healthy" individuals. Arthritis Rheum. 1997 Sep;40(9):1601–1611. doi: 10.1002/art.1780400909. [DOI] [PubMed] [Google Scholar]
- Tan E. M., Smolen J. S., McDougal J. S., Butcher B. T., Conn D., Dawkins R., Fritzler M. J., Gordon T., Hardin J. A., Kalden J. R. A critical evaluation of enzyme immunoassays for detection of antinuclear autoantibodies of defined specificities. I. Precision, sensitivity, and specificity. Arthritis Rheum. 1999 Mar;42(3):455–464. doi: 10.1002/1529-0131(199904)42:3<455::AID-ANR10>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- von Mühlen C. A., Tan E. M. Autoantibodies in the diagnosis of systemic rheumatic diseases. Semin Arthritis Rheum. 1995 Apr;24(5):323–358. doi: 10.1016/s0049-0172(95)80004-2. [DOI] [PubMed] [Google Scholar]