Abstract
OBJECTIVE—To quantify N-acetylaspartate (NAA), total creatines (tCr), total cholines (tCho), and myo-inositol (mI) levels in normal and abnormal appearing white matter of patients with neuropsychiatric systemic lupus erythematosus (NPSLE) in order to determine the specific changes in metabolite concentrations. METHODS—Axial proton density and T2 weighted magnetic resonance images, and short echo time (TE 30 ms) 1H spectra were acquired with a GE SIGNA 1.5 T magnetic resonance system. Concentrations of NAA, tCr, tCho, and mI were determined, using brain tissue water as a reference, from nine patients (seven female, mean age 40.3 years, range 16-65) with NPSLE and eight healthy women (mean age 43 years, range 31-65). RESULTS—A significant rise of tCho (12.4%, p<0.05) and mI (31.4%, p<0.005) and a significant reduction in NAA (−12%, p<0.05) was found in normal appearing white matter compared with controls. Analysis according to severity of the clinical NPSLE features (subgrouped as major or minor) showed that SLE major had reduced NAA compared with SLE minor (−18.4%, p<0.05) and controls (−20%, p<0.005). The SLE major group showed a significant rise of mI (32%, p<0.01) and the SLE minor group a significant increase in tCho (18.6%, p<0.05) compared with controls. Longitudinal analysis of brain metabolites in normal appearing white matter showed consistent abnormalities in NAA, tCho, and mI in a patient with stable clinical features and a constant rise of tCho, but transient rise of mI was seen during a flare of disease in another patient. CONCLUSION—Quantitative 1H magnetic resonance spectroscopy (MRS) suggests a particular course of neurometabolite changes that precedes irreversible reductions in NAA and permanent neuronal loss. Initially, in patients with SLE minor, there is a significant rise in tCho and a trend (reversible) for mI also to be raised. In patients with SLE major the NAA is significantly and permanently reduced and mI is significantly raised, whereas the tCho levels are near normal. Further investigations are needed to determine how specific MRS is as a clinical marker for brain disturbance in SLE.
Full Text
The Full Text of this article is available as a PDF (150.3 KB).
Figure 1 .
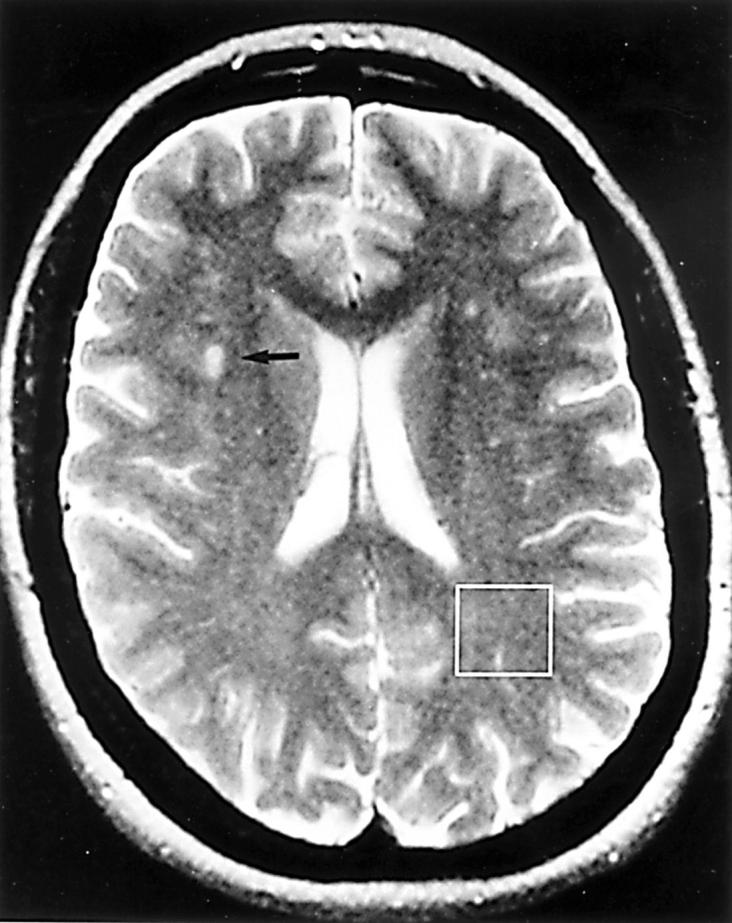
T2 weighted image showing several small foci of increased signal intensity in the white matter of both cerebral hemispheres. The largest of these is indicated by an arrow. The box represents a voxel from which 1H spectra were obtained.
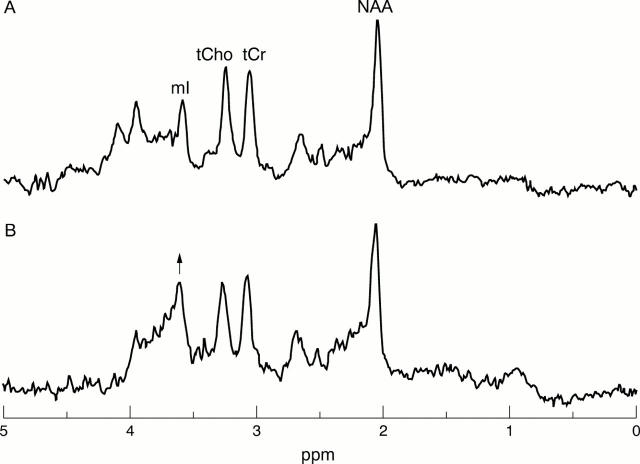
Figure 2 .
Magnetic resonance spectra from patient 6 at months 1 (A) and 18 (B) showing raised levels of tCho and mI at month 18 in comparison with month 1. (Note: the spectral changes observed above 4 ppm are not significant because this region of the spectrum is distorted by the techniques of water suppression and removal of residual water during data processing.)
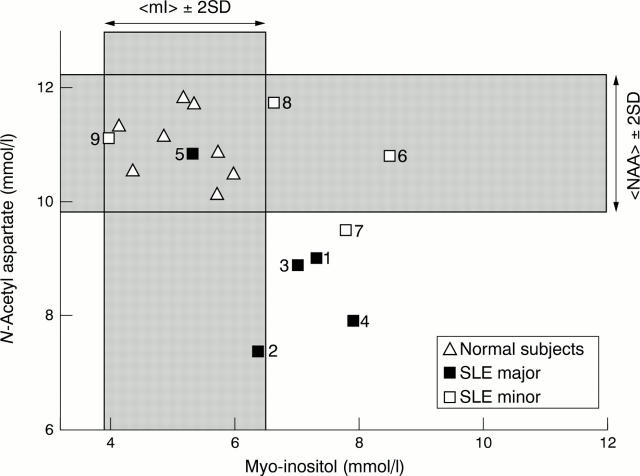
Figure 3 .
Relation between N-acetylaspartate (NAA) and myo-inositol (mI) levels in patients with major and minor features of neuropsychiatric systemic lupus erythematosus and normal subjects.
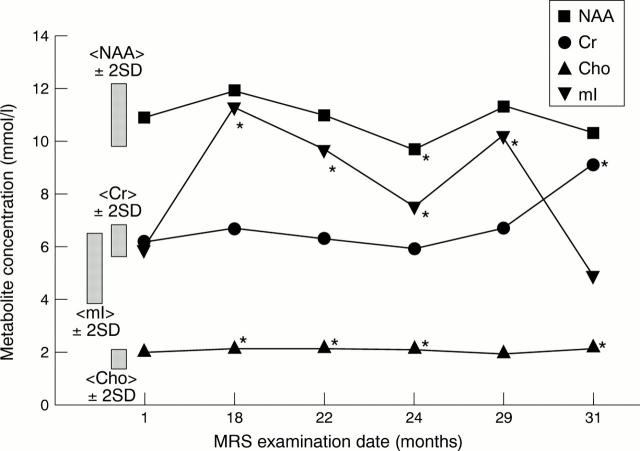
Figure 4 .
Longitudinal analysis of brain metabolites from patient No 6. This patient developed a flare of disease between months 6 and 27. During this time myo-inositol (mI) levels became significantly abnormal and reduced to baseline after treatment with a course of intravenous immunoglobulin. NAA = N-acetylaspartate; Cr = creatines; Cho = cholines.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ala-Korpela M., Usenius J. P., Keisala J., van den Boogaart A., Vainio P., Jokisaari J., Soimakallio S., Kauppinen R. Quantification of metabolites from single-voxel in vivo 1H NMR data of normal human brain by means of time-domain data analysis. MAGMA. 1995 Sep-Dec;3(3-4):129–136. doi: 10.1007/BF01771697. [DOI] [PubMed] [Google Scholar]
- Barker P. B., Soher B. J., Blackband S. J., Chatham J. C., Mathews V. P., Bryan R. N. Quantitation of proton NMR spectra of the human brain using tissue water as an internal concentration reference. NMR Biomed. 1993 Jan-Feb;6(1):89–94. doi: 10.1002/nbm.1940060114. [DOI] [PubMed] [Google Scholar]
- Bell C. L., Partington C., Robbins M., Graziano F., Turski P., Kornguth S. Magnetic resonance imaging of central nervous system lesions in patients with lupus erythematosus. Correlation with clinical remission and antineurofilament and anticardiolipin antibody titers. Arthritis Rheum. 1991 Apr;34(4):432–441. doi: 10.1002/art.1780340408. [DOI] [PubMed] [Google Scholar]
- Belmont H. M., Abramson S. B., Lie J. T. Pathology and pathogenesis of vascular injury in systemic lupus erythematosus. Interactions of inflammatory cells and activated endothelium. Arthritis Rheum. 1996 Jan;39(1):9–22. doi: 10.1002/art.1780390103. [DOI] [PubMed] [Google Scholar]
- Bourke B. E. Central nervous system involvement in systemic lupus erythematosus. Are we any further forward? Br J Rheumatol. 1993 Apr;32(4):267–268. doi: 10.1093/rheumatology/32.4.267. [DOI] [PubMed] [Google Scholar]
- Brenner R. E., Munro P. M., Williams S. C., Bell J. D., Barker G. J., Hawkins C. P., Landon D. N., McDonald W. I. The proton NMR spectrum in acute EAE: the significance of the change in the Cho:Cr ratio. Magn Reson Med. 1993 Jun;29(6):737–745. doi: 10.1002/mrm.1910290605. [DOI] [PubMed] [Google Scholar]
- Brooks W. M., Jung R. E., Ford C. C., Greinel E. J., Sibbitt W. L., Jr Relationship between neurometabolite derangement and neurocognitive dysfunction in systemic lupus erythematosus. J Rheumatol. 1999 Jan;26(1):81–85. [PubMed] [Google Scholar]
- Brooks W. M., Sabet A., Sibbitt W. L., Jr, Barker P. B., van Zijl P. C., Duyn J. H., Moonen C. T. Neurochemistry of brain lesions determined by spectroscopic imaging in systemic lupus erythematosus. J Rheumatol. 1997 Dec;24(12):2323–2329. [PubMed] [Google Scholar]
- Bruyn G. A. Controversies in lupus: nervous system involvement. Ann Rheum Dis. 1995 Mar;54(3):159–167. doi: 10.1136/ard.54.3.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cauli A., Montaldo C., Peltz M. T., Nurchis P., Sanna G., Garau P., Pala R., Passiu G., Mathieu A. Abnormalities of magnetic resonance imaging of the central nervous system in patients with systemic lupus erythematosus correlate with disease severity. Clin Rheumatol. 1994 Dec;13(4):615–618. doi: 10.1007/BF02243004. [DOI] [PubMed] [Google Scholar]
- Chinn R. J., Wilkinson I. D., Hall-Craggs M. A., Paley M. N., Shortall E., Carter S., Kendall B. E., Isenberg D. A., Newman S. P., Harrison M. J. Magnetic resonance imaging of the brain and cerebral proton spectroscopy in patients with systemic lupus erythematosus. Arthritis Rheum. 1997 Jan;40(1):36–46. doi: 10.1002/art.1780400107. [DOI] [PubMed] [Google Scholar]
- Davie C. A., Feinstein A., Kartsounis L. D., Barker G. J., McHugh N. J., Walport M. J., Ron M. A., Moseley I. F., McDonald W. I., Miller D. H. Proton magnetic resonance spectroscopy of systemic lupus erythematosus involving the central nervous system. J Neurol. 1995 Aug;242(8):522–528. doi: 10.1007/BF00867424. [DOI] [PubMed] [Google Scholar]
- Denburg S. D., Behmann S. A., Carbotte R. M., Denburg J. A. Lymphocyte antigens in neuropsychiatric systemic lupus erythematosus. Relationship of lymphocyte antibody specificities to clinical disease. Arthritis Rheum. 1994 Mar;37(3):369–375. doi: 10.1002/art.1780370310. [DOI] [PubMed] [Google Scholar]
- Ernst T., Chang L., Melchor R., Mehringer C. M. Frontotemporal dementia and early Alzheimer disease: differentiation with frontal lobe H-1 MR spectroscopy. Radiology. 1997 Jun;203(3):829–836. doi: 10.1148/radiology.203.3.9169712. [DOI] [PubMed] [Google Scholar]
- Friedman S. D., Stidley C. A., Brooks W. M., Hart B. L., Sibbitt W. L., Jr Brain injury and neurometabolic abnormalities in systemic lupus erythematosus. Radiology. 1998 Oct;209(1):79–84. doi: 10.1148/radiology.209.1.9769816. [DOI] [PubMed] [Google Scholar]
- Hanly J. G., Fisk J. D., Sherwood G., Jones E., Jones J. V., Eastwood B. Cognitive impairment in patients with systemic lupus erythematosus. J Rheumatol. 1992 Apr;19(4):562–567. [PubMed] [Google Scholar]
- Häussinger D., Laubenberger J., vom Dahl S., Ernst T., Bayer S., Langer M., Gerok W., Hennig J. Proton magnetic resonance spectroscopy studies on human brain myo-inositol in hypo-osmolarity and hepatic encephalopathy. Gastroenterology. 1994 Nov;107(5):1475–1480. doi: 10.1016/0016-5085(94)90552-5. [DOI] [PubMed] [Google Scholar]
- Jarek M. J., West S. G., Baker M. R., Rak K. M. Magnetic resonance imaging in systemic lupus erythematosus patients without a history of neuropsychiatric lupus erythematosus. Arthritis Rheum. 1994 Nov;37(11):1609–1613. doi: 10.1002/art.1780371108. [DOI] [PubMed] [Google Scholar]
- Klose U. In vivo proton spectroscopy in presence of eddy currents. Magn Reson Med. 1990 Apr;14(1):26–30. doi: 10.1002/mrm.1910140104. [DOI] [PubMed] [Google Scholar]
- Koopmans R. A., Li D. K., Zhu G., Allen P. S., Penn A., Paty D. W. Magnetic resonance spectroscopy of multiple sclerosis: in-vivo detection of myelin breakdown products. Lancet. 1993 Mar 6;341(8845):631–632. doi: 10.1016/0140-6736(93)90391-s. [DOI] [PubMed] [Google Scholar]
- Kreis R., Ernst T., Ross B. D. Development of the human brain: in vivo quantification of metabolite and water content with proton magnetic resonance spectroscopy. Magn Reson Med. 1993 Oct;30(4):424–437. doi: 10.1002/mrm.1910300405. [DOI] [PubMed] [Google Scholar]
- Kreis R., Ross B. D. Cerebral metabolic disturbances in patients with subacute and chronic diabetes mellitus: detection with proton MR spectroscopy. Radiology. 1992 Jul;184(1):123–130. doi: 10.1148/radiology.184.1.1319074. [DOI] [PubMed] [Google Scholar]
- Lee J. H., Arcinue E., Ross B. D. Brief report: organic osmolytes in the brain of an infant with hypernatremia. N Engl J Med. 1994 Aug 18;331(7):439–442. doi: 10.1056/NEJM199408183310704. [DOI] [PubMed] [Google Scholar]
- López-Villegas D., Lenkinski R. E., Frank I. Biochemical changes in the frontal lobe of HIV-infected individuals detected by magnetic resonance spectroscopy. Proc Natl Acad Sci U S A. 1997 Sep 2;94(18):9854–9859. doi: 10.1073/pnas.94.18.9854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michaelis T., Merboldt K. D., Bruhn H., Hänicke W., Frahm J. Absolute concentrations of metabolites in the adult human brain in vivo: quantification of localized proton MR spectra. Radiology. 1993 Apr;187(1):219–227. doi: 10.1148/radiology.187.1.8451417. [DOI] [PubMed] [Google Scholar]
- Molad Y., Sidi Y., Gornish M., Lerner M., Pinkhas J., Weinberger A. Lupus anticoagulant: correlation with magnetic resonance imaging of brain lesions. J Rheumatol. 1992 Apr;19(4):556–561. [PubMed] [Google Scholar]
- Sabet A., Sibbitt W. L., Jr, Stidley C. A., Danska J., Brooks W. M. Neurometabolite markers of cerebral injury in the antiphospholipid antibody syndrome of systemic lupus erythematosus. Stroke. 1998 Nov;29(11):2254–2260. doi: 10.1161/01.str.29.11.2254. [DOI] [PubMed] [Google Scholar]
- Saunders D. E., Howe F. A., van den Boogaart A., Griffiths J. R., Brown M. M. Aging of the adult human brain: in vivo quantitation of metabolite content with proton magnetic resonance spectroscopy. J Magn Reson Imaging. 1999 May;9(5):711–716. doi: 10.1002/(sici)1522-2586(199905)9:5<711::aid-jmri14>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- Saunders D. E., Howe F. A., van den Boogaart A., McLean M. A., Griffiths J. R., Brown M. M. Continuing ischemic damage after acute middle cerebral artery infarction in humans demonstrated by short-echo proton spectroscopy. Stroke. 1995 Jun;26(6):1007–1013. doi: 10.1161/01.str.26.6.1007. [DOI] [PubMed] [Google Scholar]
- Shonk T., Ross B. D. Role of increased cerebral myo-inositol in the dementia of Down syndrome. Magn Reson Med. 1995 Jun;33(6):858–861. doi: 10.1002/mrm.1910330619. [DOI] [PubMed] [Google Scholar]
- Sibbitt W. L., Jr, Brooks W. M., Haseler L. J., Griffey R. H., Frank L. M., Hart B. L., Sibbitt R. R. Spin-spin relaxation of brain tissues in systemic lupus erythematosus. A method for increasing the sensitivity of magnetic resonance imaging for neuropsychiatric lupus. Arthritis Rheum. 1995 Jun;38(6):810–818. doi: 10.1002/art.1780380615. [DOI] [PubMed] [Google Scholar]
- Sibbitt W. L., Jr, Haseler L. J., Griffey R. H., Hart B. L., Sibbitt R. R., Matwiyoff N. A. Analysis of cerebral structural changes in systemic lupus erythematosus by proton MR spectroscopy. AJNR Am J Neuroradiol. 1994 May;15(5):923–928. [PMC free article] [PubMed] [Google Scholar]
- Sibbitt W. L., Jr, Haseler L. J., Griffey R. R., Friedman S. D., Brooks W. M. Neurometabolism of active neuropsychiatric lupus determined with proton MR spectroscopy. AJNR Am J Neuroradiol. 1997 Aug;18(7):1271–1277. [PMC free article] [PubMed] [Google Scholar]
- Simmons A., Smail M., Moore E., Williams S. C. Serial precision of metabolite peak area ratios and water referenced metabolite peak areas in proton MR spectroscopy of the human brain. Magn Reson Imaging. 1998 Apr;16(3):319–330. doi: 10.1016/s0730-725x(97)00280-4. [DOI] [PubMed] [Google Scholar]
- Soher B. J., Hurd R. E., Sailasuta N., Barker P. B. Quantitation of automated single-voxel proton MRS using cerebral water as an internal reference. Magn Reson Med. 1996 Sep;36(3):335–339. doi: 10.1002/mrm.1910360302. [DOI] [PubMed] [Google Scholar]
- Stanley J. A., Drost D. J., Williamson P. C., Thompson R. T. The use of a priori knowledge to quantify short echo in vivo 1H MR spectra. Magn Reson Med. 1995 Jul;34(1):17–24. doi: 10.1002/mrm.1910340105. [DOI] [PubMed] [Google Scholar]
- Stimmler M. M., Coletti P. M., Quismorio F. P., Jr Magnetic resonance imaging of the brain in neuropsychiatric systemic lupus erythematosus. Semin Arthritis Rheum. 1993 Apr;22(5):335–349. doi: 10.1016/s0049-0172(05)80012-5. [DOI] [PubMed] [Google Scholar]
- Tan E. M., Cohen A. S., Fries J. F., Masi A. T., McShane D. J., Rothfield N. F., Schaller J. G., Talal N., Winchester R. J. The 1982 revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum. 1982 Nov;25(11):1271–1277. doi: 10.1002/art.1780251101. [DOI] [PubMed] [Google Scholar]
- Videen J. S., Michaelis T., Pinto P., Ross B. D. Human cerebral osmolytes during chronic hyponatremia. A proton magnetic resonance spectroscopy study. J Clin Invest. 1995 Feb;95(2):788–793. doi: 10.1172/JCI117728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webb P. G., Sailasuta N., Kohler S. J., Raidy T., Moats R. A., Hurd R. E. Automated single-voxel proton MRS: technical development and multisite verification. Magn Reson Med. 1994 Apr;31(4):365–373. doi: 10.1002/mrm.1910310404. [DOI] [PubMed] [Google Scholar]
- West S. G., Emlen W., Wener M. H., Kotzin B. L. Neuropsychiatric lupus erythematosus: a 10-year prospective study on the value of diagnostic tests. Am J Med. 1995 Aug;99(2):153–163. doi: 10.1016/s0002-9343(99)80135-1. [DOI] [PubMed] [Google Scholar]
- van der Veen J. W., de Beer R., Luyten P. R., van Ormondt D. Accurate quantification of in vivo 31P NMR signals using the variable projection method and prior knowledge. Magn Reson Med. 1988 Jan;6(1):92–98. doi: 10.1002/mrm.1910060111. [DOI] [PubMed] [Google Scholar]