Abstract
OBJECTIVE—To investigate whether interleukin 13 (IL13) could act in a chondroprotective manner and protect cartilage stimulated to resorb with a combination of IL1α and oncostatin M (OSM), in a similar way to the anti-inflammatory cytokine, IL4. METHODS—IL13 was added to explant cultures of bovine nasal cartilage stimulated to resorb with IL1α and OSM, and the release of collagen and proteoglycan determined. Collagenolytic and tissue inhibitors of metalloproteinase (TIMP) activities were determined by bioassay. Northern blot analyses were performed to determine the effects of IL13 on the induction of matrix metalloproteinase-1 (MMP-1), MMP-3, MMP-13, and TIMP-1 gene expression. RESULTS—IL13 can prevent the release of collagen from bovine nasal cartilage in a dose dependent manner. This was accompanied by a concomitant decrease in measurable collagenolytic activity in the culture supernates and an increase in TIMP activity. Northern blot analysis showed that IL13 down regulated MMP-3 and MMP-13 levels but up regulated MMP-1 and TIMP-1 gene expression in bovine nasal chondrocytes at 24 hours. CONCLUSION—This study showed for the first time that IL13 can block collagen release from resorbing cartilage in a similar manner to IL4. This is accompanied by a reduction in detectable collagenolytic activity, a decrease in MMP-3 and MMP-13 mRNA levels, and an up regulation of TIMP-1 expression.
Full Text
The Full Text of this article is available as a PDF (186.2 KB).
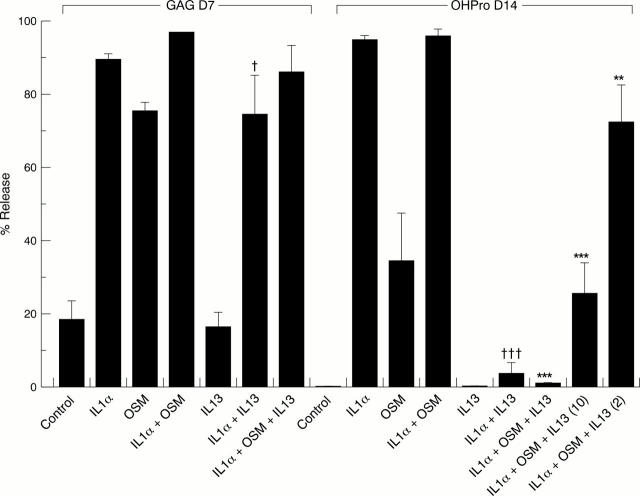
Figure 1 .
The effect of interleukin 1α (IL1α) in combination with oncostatin M (OSM), with or without IL13, on the release of proteoglycan and collagen from bovine nasal cartilage in explant culture. Three discs of cartilage per well in quadruplicate were cultured in 600 µl control medium alone, IL1α (1 ng/ml), OSM (10 ng/ml), IL1α + OSM, with and without IL13 (2-50 ng/ml), for 0-7 days and the media removed. Each well was replenished under identical conditions and left for a further seven days. At day 14 media were removed and the remaining cartilage digested with papain. The levels of glycosaminoglycan (GAG) and hydroxyproline (OHPro) released into the medium on days 7 and 14 were determined and the results expressed as a percentage of the total. Results are expressed as mean (SD). The data shown are representative of three independent experiments. Student's unpaired two tailed t test was used to compare IL1α and IL1α + IL13, where †††p<0.001 and †p<0.05. The same test was used to compare IL1α + OSM with IL1α + OSM + IL13, where ***p<0.001 and **p<0.01.
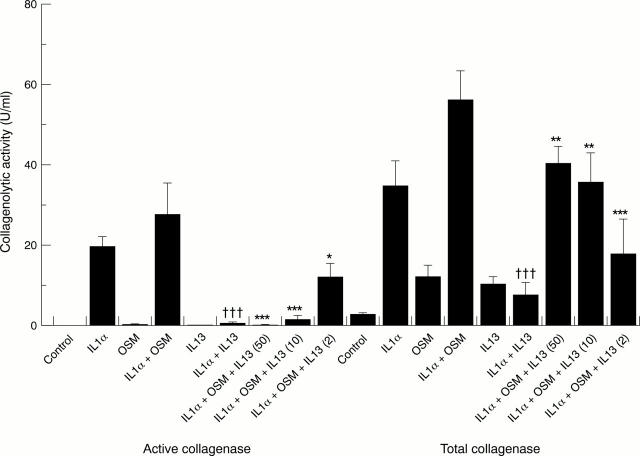
Figure 2 .
Levels of active and total collagenase activity in media samples removed from cartilage cultures at day 14 after stimulation with interleukin 1α (IL1α), oncostatin M (OSM), IL1α + OSM with and without IL13 (50 ng/ml). Cartilage was incubated and treated as described in fig 1. The levels of active and total collagenase released into the medium on day 14 were measured and the results expressed as U/ml (mean (SD)). Active and total collagenase were measured as described in "Materials and methods". Student's unpaired two tailed t test was used to compare IL1α and IL1α + IL13, where †††p<0.001. The same test was used to compare IL1α + OSM with IL1α + OSM + IL13, where ***p<0.001, **p<0.01, and *p<0.05.
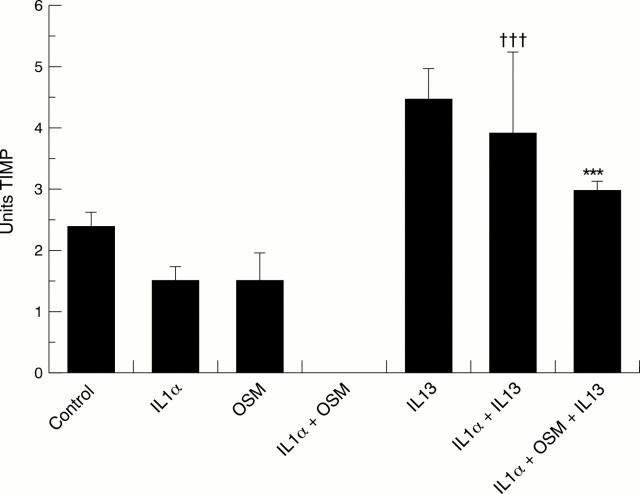
Figure 3 .
Levels of tissue inhibitors of metalloproteinase (TIMP) inhibitory activity in media samples removed from cartilage cultures at day 14 after stimulation with interleukin 1α ( IL1α), oncostatin M (OSM), IL1α + OSM, with and without IL13. Cartilage was incubated and treated as described in fig 1. The levels of TIMP released into the medium on day 14 were measured and the results expressed as U/ml (mean (SD)). Student's unpaired two tailed t test was used to compare IL1α and IL1α + IL13, where †††p<0.001. The same test was used to compare IL1α + OSM with IL1α + OSM + IL13, where ***p<0.001.
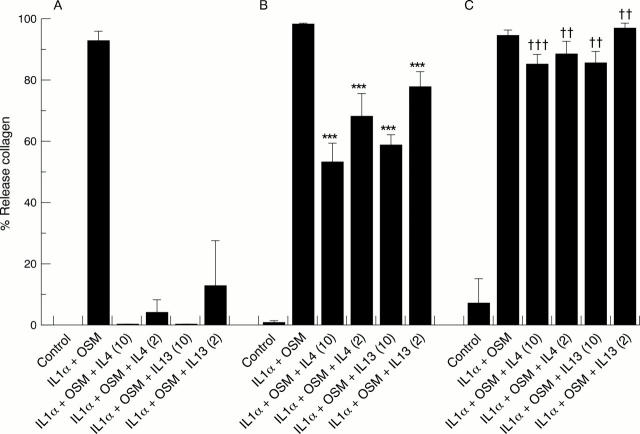
Figure 4 .
Cartilage collagen release inhibited by interleukin 13 (IL13) and IL4 can be recovered by addition of an exogenous activator of procollagenases. Three discs of cartilage per well in quadruplicate were cultured in 600 µl control medium alone, IL1α (1 ng/ml) + oncostatin M (OSM) (10 ng/ml), with and without IL4/13 (2-50 ng/ml), for 0-7 days, and the media removed. Media containing identical cytokine combinations to day 1 were replenished. One set received no further additions (A), whereas others had exogenous active matrix metalloproteinase-3 (MMP-3) added at 0.83 µg/ml (B), or 3.33 µg/ml (C). All cultures were left for a further seven days. At day 14 media were removed and the remaining cartilage digested with papain. The levels of hydroxyproline (OHPro) released into the medium on days 7 and 14 were determined and the results expressed as a percentage of the total released. Results are expressed as mean (SD). The data shown are representative of three independent experiments. Student's unpaired two tailed t test was used to analyse data, firstly, where the addition of MMP-3 at 0.83 µg/ml (B) was compared with its identical treatment in the absence of exogenous MMP-3 (A), where ***p<0.001, and, secondly, where the addition of MMP-3 at 3.33 µg/ml (C) was compared with its identical treatment in the presence of MMP-3 at 0.83 µg/ml (B), where †††p<0.001 and ††p<0.01.
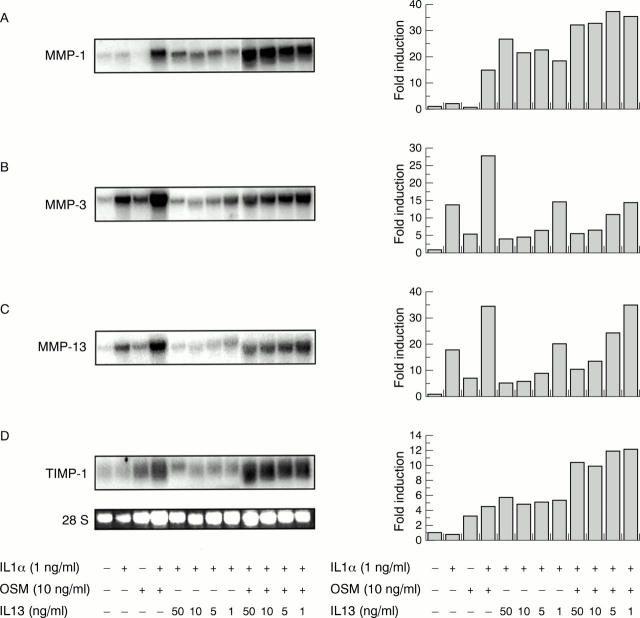
Figure 5 .
Interleukin 13 (IL13) inhibits IL1α and IL1α + oncostatin M (OSM) induction of metalloproteinase-3 (MMP-3) and MMP-13 mRNA but not MMP-1 or tissue inhibitor of matrix metalloproteinase-1 (TIMP-1) mRNA in bovine nasal chondrocytes at 24 hours. Total RNA was isolated at 24 hours after the addition of IL1α (1 ng/ml), OSM (10 ng/ml), IL1α + OSM, alone or in combination with IL13 in cultures of bovine nasal chondrocytes. Northern blots were hybridised to cDNA probes corresponding to MMP-1 (A), MMP-3 (B), MMP-13 (C), and TIMP-1 (D). Ethidium bromide stained 28 S rRNA was used for normalisation of RNA loading. The northern blots are shown on the left hand side of the figure and were measured by scanning densitometry. The results are expressed as fold induction over control on the right hand side. The data shown are representative of three independent experiments.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abbaszade I., Liu R. Q., Yang F., Rosenfeld S. A., Ross O. H., Link J. R., Ellis D. M., Tortorella M. D., Pratta M. A., Hollis J. M. Cloning and characterization of ADAMTS11, an aggrecanase from the ADAMTS family. J Biol Chem. 1999 Aug 13;274(33):23443–23450. doi: 10.1074/jbc.274.33.23443. [DOI] [PubMed] [Google Scholar]
- Agren M. S., Taplin C. J., Woessner J. F., Jr, Eaglstein W. H., Mertz P. M. Collagenase in wound healing: effect of wound age and type. J Invest Dermatol. 1992 Dec;99(6):709–714. doi: 10.1111/1523-1747.ep12614202. [DOI] [PubMed] [Google Scholar]
- Aimes R. T., Quigley J. P. Matrix metalloproteinase-2 is an interstitial collagenase. Inhibitor-free enzyme catalyzes the cleavage of collagen fibrils and soluble native type I collagen generating the specific 3/4- and 1/4-length fragments. J Biol Chem. 1995 Mar 17;270(11):5872–5876. doi: 10.1074/jbc.270.11.5872. [DOI] [PubMed] [Google Scholar]
- Apte S. S., Mattei M. G., Olsen B. R. Cloning of the cDNA encoding human tissue inhibitor of metalloproteinases-3 (TIMP-3) and mapping of the TIMP3 gene to chromosome 22. Genomics. 1994 Jan 1;19(1):86–90. doi: 10.1006/geno.1994.1016. [DOI] [PubMed] [Google Scholar]
- Arend W. P., Malyak M., Guthridge C. J., Gabay C. Interleukin-1 receptor antagonist: role in biology. Annu Rev Immunol. 1998;16:27–55. doi: 10.1146/annurev.immunol.16.1.27. [DOI] [PubMed] [Google Scholar]
- Bessis N., Boissier M. C., Ferrara P., Blankenstein T., Fradelizi D., Fournier C. Attenuation of collagen-induced arthritis in mice by treatment with vector cells engineered to secrete interleukin-13. Eur J Immunol. 1996 Oct;26(10):2399–2403. doi: 10.1002/eji.1830261020. [DOI] [PubMed] [Google Scholar]
- Billington C. J., Clark I. M., Cawston T. E. An aggrecan-degrading activity associated with chondrocyte membranes. Biochem J. 1998 Nov 15;336(Pt 1):207–212. doi: 10.1042/bj3360207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borghaei R. C., Rawlings P. L., Jr, Mochan E. Interleukin-4 suppression of interleukin-1-induced transcription of collagenase (MMP-1) and stromelysin 1 (MMP-3) in human synovial fibroblasts. Arthritis Rheum. 1998 Aug;41(8):1398–1406. doi: 10.1002/1529-0131(199808)41:8<1398::AID-ART8>3.0.CO;2-B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brinckerhoff C. E. Joint destruction in arthritis: metalloproteinases in the spotlight. Arthritis Rheum. 1991 Sep;34(9):1073–1075. doi: 10.1002/art.1780340902. [DOI] [PubMed] [Google Scholar]
- Cawston T. E., Curry V. A., Summers C. A., Clark I. M., Riley G. P., Life P. F., Spaull J. R., Goldring M. B., Koshy P. J., Rowan A. D. The role of oncostatin M in animal and human connective tissue collagen turnover and its localization within the rheumatoid joint. Arthritis Rheum. 1998 Oct;41(10):1760–1771. doi: 10.1002/1529-0131(199810)41:10<1760::AID-ART8>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- Cawston T. E., Ellis A. J., Bigg H., Curry V., Lean E., Ward D. Interleukin-4 blocks the release of collagen fragments from bovine nasal cartilage treated with cytokines. Biochim Biophys Acta. 1996 Dec 12;1314(3):226–232. doi: 10.1016/s0167-4889(96)00107-3. [DOI] [PubMed] [Google Scholar]
- Cawston T. E., Ellis A. J., Humm G., Lean E., Ward D., Curry V. Interleukin-1 and oncostatin M in combination promote the release of collagen fragments from bovine nasal cartilage in culture. Biochem Biophys Res Commun. 1995 Oct 4;215(1):377–385. doi: 10.1006/bbrc.1995.2476. [DOI] [PubMed] [Google Scholar]
- Clark I. M., Powell L. K., Ramsey S., Hazleman B. L., Cawston T. E. The measurement of collagenase, tissue inhibitor of metalloproteinases (TIMP), and collagenase-TIMP complex in synovial fluids from patients with osteoarthritis and rheumatoid arthritis. Arthritis Rheum. 1993 Mar;36(3):372–379. doi: 10.1002/art.1780360313. [DOI] [PubMed] [Google Scholar]
- Defrance T., Carayon P., Billian G., Guillemot J. C., Minty A., Caput D., Ferrara P. Interleukin 13 is a B cell stimulating factor. J Exp Med. 1994 Jan 1;179(1):135–143. doi: 10.1084/jem.179.1.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dingle J. T., Page Thomas D. P., King B., Bard D. R. In vivo studies of articular tissue damage mediated by catabolin/interleukin 1. Ann Rheum Dis. 1987 Jul;46(7):527–533. doi: 10.1136/ard.46.7.527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Docherty A. J., Lyons A., Smith B. J., Wright E. M., Stephens P. E., Harris T. J., Murphy G., Reynolds J. J. Sequence of human tissue inhibitor of metalloproteinases and its identity to erythroid-potentiating activity. Nature. 1985 Nov 7;318(6041):66–69. doi: 10.1038/318066a0. [DOI] [PubMed] [Google Scholar]
- Doherty T. M., Kastelein R., Menon S., Andrade S., Coffman R. L. Modulation of murine macrophage function by IL-13. J Immunol. 1993 Dec 15;151(12):7151–7160. [PubMed] [Google Scholar]
- Farndale R. W., Buttle D. J., Barrett A. J. Improved quantitation and discrimination of sulphated glycosaminoglycans by use of dimethylmethylene blue. Biochim Biophys Acta. 1986 Sep 4;883(2):173–177. doi: 10.1016/0304-4165(86)90306-5. [DOI] [PubMed] [Google Scholar]
- Freije J. M., Díez-Itza I., Balbín M., Sánchez L. M., Blasco R., Tolivia J., López-Otín C. Molecular cloning and expression of collagenase-3, a novel human matrix metalloproteinase produced by breast carcinomas. J Biol Chem. 1994 Jun 17;269(24):16766–16773. [PubMed] [Google Scholar]
- Greene J., Wang M., Liu Y. E., Raymond L. A., Rosen C., Shi Y. E. Molecular cloning and characterization of human tissue inhibitor of metalloproteinase 4. J Biol Chem. 1996 Nov 29;271(48):30375–30380. doi: 10.1074/jbc.271.48.30375. [DOI] [PubMed] [Google Scholar]
- Hamilton J. A., Leizer T., Piccoli D. S., Royston K. M., Butler D. M., Croatto M. Oncostatin M stimulates urokinase-type plasminogen activator activity in human synovial fibroblasts. Biochem Biophys Res Commun. 1991 Oct 31;180(2):652–659. doi: 10.1016/s0006-291x(05)81115-5. [DOI] [PubMed] [Google Scholar]
- Harris E. D., Jr, DiBona D. R., Krane S. M. Collagenases in human synovial fluid. J Clin Invest. 1969 Nov;48(11):2104–2113. doi: 10.1172/JCI106177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasty K. A., Jeffrey J. J., Hibbs M. S., Welgus H. G. The collagen substrate specificity of human neutrophil collagenase. J Biol Chem. 1987 Jul 25;262(21):10048–10052. [PubMed] [Google Scholar]
- Iannone F., Corrigall V. M., Kingsley G. H., Panayi G. S. Evidence for the continuous recruitment and activation of T cells into the joints of patients with rheumatoid arthritis. Eur J Immunol. 1994 Nov;24(11):2706–2713. doi: 10.1002/eji.1830241120. [DOI] [PubMed] [Google Scholar]
- Isomäki P., Luukkainen R., Toivanen P., Punnonen J. The presence of interleukin-13 in rheumatoid synovium and its antiinflammatory effects on synovial fluid macrophages from patients with rheumatoid arthritis. Arthritis Rheum. 1996 Oct;39(10):1693–1702. doi: 10.1002/art.1780391012. [DOI] [PubMed] [Google Scholar]
- Jenkins J. K., Malyak M., Arend W. P. The effects of interleukin-10 on interleukin-1 receptor antagonist and interleukin-1 beta production in human monocytes and neutrophils. Lymphokine Cytokine Res. 1994 Feb;13(1):47–54. [PubMed] [Google Scholar]
- Jovanovic D., Pelletier J. P., Alaaeddine N., Mineau F., Geng C., Ranger P., Martel-Pelletier J. Effect of IL-13 on cytokines, cytokine receptors and inhibitors on human osteoarthritis synovium and synovial fibroblasts. Osteoarthritis Cartilage. 1998 Jan;6(1):40–49. doi: 10.1053/joca.1997.0091. [DOI] [PubMed] [Google Scholar]
- Jubb R. W., Fell H. B. The breakdown of collagen by chondrocytes. J Pathol. 1980 Mar;130(3):159–167. doi: 10.1002/path.1711300304. [DOI] [PubMed] [Google Scholar]
- Koshy P. J., Rowan A. D., Life P. F., Cawston T. E. 96-Well plate assays for measuring collagenase activity using (3)H-acetylated collagen. Anal Biochem. 1999 Nov 15;275(2):202–207. doi: 10.1006/abio.1999.4310. [DOI] [PubMed] [Google Scholar]
- Matrisian L. M. The matrix-degrading metalloproteinases. Bioessays. 1992 Jul;14(7):455–463. doi: 10.1002/bies.950140705. [DOI] [PubMed] [Google Scholar]
- Miller E. J., Harris E. D., Jr, Chung E., Finch J. E., Jr, McCroskery P. A., Butler W. T. Cleavage of Type II and III collagens with mammalian collagenase: site of cleavage and primary structure at the NH2-terminal portion of the smaller fragment released from both collagens. Biochemistry. 1976 Feb 24;15(4):787–792. doi: 10.1021/bi00649a009. [DOI] [PubMed] [Google Scholar]
- Miltenburg A. M., van Laar J. M., de Kuiper R., Daha M. R., Breedveld F. C. T cells cloned from human rheumatoid synovial membrane functionally represent the Th1 subset. Scand J Immunol. 1992 May;35(5):603–610. doi: 10.1111/j.1365-3083.1992.tb03260.x. [DOI] [PubMed] [Google Scholar]
- Minty A., Chalon P., Derocq J. M., Dumont X., Guillemot J. C., Kaghad M., Labit C., Leplatois P., Liauzun P., Miloux B. Interleukin-13 is a new human lymphokine regulating inflammatory and immune responses. Nature. 1993 Mar 18;362(6417):248–250. doi: 10.1038/362248a0. [DOI] [PubMed] [Google Scholar]
- Murphy G., Cockett M. I., Stephens P. E., Smith B. J., Docherty A. J. Stromelysin is an activator of procollagenase. A study with natural and recombinant enzymes. Biochem J. 1987 Nov 15;248(1):265–268. doi: 10.1042/bj2480265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy G., Hembry R. M. Proteinases in rheumatoid arthritis. J Rheumatol Suppl. 1992 Jan;32:61–64. [PubMed] [Google Scholar]
- Ohuchi E., Imai K., Fujii Y., Sato H., Seiki M., Okada Y. Membrane type 1 matrix metalloproteinase digests interstitial collagens and other extracellular matrix macromolecules. J Biol Chem. 1997 Jan 24;272(4):2446–2451. doi: 10.1074/jbc.272.4.2446. [DOI] [PubMed] [Google Scholar]
- Onoe Y., Miyaura C., Kaminakayashiki T., Nagai Y., Noguchi K., Chen Q. R., Seo H., Ohta H., Nozawa S., Kudo I. IL-13 and IL-4 inhibit bone resorption by suppressing cyclooxygenase-2-dependent prostaglandin synthesis in osteoblasts. J Immunol. 1996 Jan 15;156(2):758–764. [PubMed] [Google Scholar]
- Page Thomas D. P., King B., Stephens T., Dingle J. T. In vivo studies of cartilage regeneration after damage induced by catabolin/interleukin-1. Ann Rheum Dis. 1991 Feb;50(2):75–80. doi: 10.1136/ard.50.2.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez R. M., Pitzalis C., Kingsley G. H., Henderson E., Humphries M. J., Panayi G. S. T lymphocyte adhesion to fibronectin (FN): a possible mechanism for T cell accumulation in the rheumatoid joint. Clin Exp Immunol. 1992 Sep;89(3):439–445. doi: 10.1111/j.1365-2249.1992.tb06977.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sah R. L., Chen A. C., Grodzinsky A. J., Trippel S. B. Differential effects of bFGF and IGF-I on matrix metabolism in calf and adult bovine cartilage explants. Arch Biochem Biophys. 1994 Jan;308(1):137–147. doi: 10.1006/abbi.1994.1020. [DOI] [PubMed] [Google Scholar]
- Saklatvala J. Tumour necrosis factor alpha stimulates resorption and inhibits synthesis of proteoglycan in cartilage. Nature. 1986 Aug 7;322(6079):547–549. doi: 10.1038/322547a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shlopov B. V., Smith G. N., Jr, Cole A. A., Hasty K. A. Differential patterns of response to doxycycline and transforming growth factor beta1 in the down-regulation of collagenases in osteoarthritic and normal human chondrocytes. Arthritis Rheum. 1999 Apr;42(4):719–727. doi: 10.1002/1529-0131(199904)42:4<719::AID-ANR15>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- Stetler-Stevenson W. G., Krutzsch H. C., Liotta L. A. Tissue inhibitor of metalloproteinase (TIMP-2). A new member of the metalloproteinase inhibitor family. J Biol Chem. 1989 Oct 15;264(29):17374–17378. [PubMed] [Google Scholar]
- Stricklin G. P., Bauer E. A., Jeffrey J. J., Eisen A. Z. Human skin collagenase: isolation of precursor and active forms from both fibroblast and organ cultures. Biochemistry. 1977 Apr 19;16(8):1607–1615. doi: 10.1021/bi00627a013. [DOI] [PubMed] [Google Scholar]
- Tortorella M. D., Burn T. C., Pratta M. A., Abbaszade I., Hollis J. M., Liu R., Rosenfeld S. A., Copeland R. A., Decicco C. P., Wynn R. Purification and cloning of aggrecanase-1: a member of the ADAMTS family of proteins. Science. 1999 Jun 4;284(5420):1664–1666. doi: 10.1126/science.284.5420.1664. [DOI] [PubMed] [Google Scholar]
- Uría J. A., Jiménez M. G., Balbín M., Freije J. M., López-Otín C. Differential effects of transforming growth factor-beta on the expression of collagenase-1 and collagenase-3 in human fibroblasts. J Biol Chem. 1998 Apr 17;273(16):9769–9777. doi: 10.1074/jbc.273.16.9769. [DOI] [PubMed] [Google Scholar]
- Vannier E., de Waal Malefyt R., Salazar-Montes A., de Vries J. E., Dinarello C. A. Interleukin-13 (IL-13) induces IL-1 receptor antagonist gene expression and protein synthesis in peripheral blood mononuclear cells: inhibition by an IL-4 mutant protein. Blood. 1996 Apr 15;87(8):3307–3315. [PubMed] [Google Scholar]
- Woessner J. F., Jr Matrix metalloproteinases and their inhibitors in connective tissue remodeling. FASEB J. 1991 May;5(8):2145–2154. [PubMed] [Google Scholar]
- Woolley D. E., Crossley M. J., Evanson J. M. Collagenase at sites of cartilage erosion in the rheumatoid joint. Arthritis Rheum. 1977 Jul-Aug;20(6):1231–1239. doi: 10.1002/art.1780200612. [DOI] [PubMed] [Google Scholar]
- Zurawski G., de Vries J. E. Interleukin 13, an interleukin 4-like cytokine that acts on monocytes and B cells, but not on T cells. Immunol Today. 1994 Jan;15(1):19–26. doi: 10.1016/0167-5699(94)90021-3. [DOI] [PubMed] [Google Scholar]
- de Waal Malefyt R., Figdor C. G., Huijbens R., Mohan-Peterson S., Bennett B., Culpepper J., Dang W., Zurawski G., de Vries J. E. Effects of IL-13 on phenotype, cytokine production, and cytotoxic function of human monocytes. Comparison with IL-4 and modulation by IFN-gamma or IL-10. J Immunol. 1993 Dec 1;151(11):6370–6381. [PubMed] [Google Scholar]
- van Meurs J., van Lent P., Stoop R., Holthuysen A., Singer I., Bayne E., Mudgett J., Poole R., Billinghurst C., van der Kraan P. Cleavage of aggrecan at the Asn341-Phe342 site coincides with the initiation of collagen damage in murine antigen-induced arthritis: a pivotal role for stromelysin 1 in matrix metalloproteinase activity. Arthritis Rheum. 1999 Oct;42(10):2074–2084. doi: 10.1002/1529-0131(199910)42:10<2074::AID-ANR7>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]