Abstract
OBJECTIVES—(1) To determine whether serum concentration of serum amyloid A (SAA) protein is influenced by the SAA1 allele in Japanese patients with rheumatoid arthritis (RA) as previously shown in a healthy control group; and (2) to analyse what factors, based on such an allelic bias, influence the relative SAA values of those patients. METHODS—SAA and C reactive protein (CRP) concentrations together with SAA1 genotypes were determined in 316 Japanese patients with RA. The relative SAA values were evaluated as an SAA/CRP ratio. RESULTS—Comparison of the three SAA1 homozygote groups showed that the SAA/CRP ratio was highest in the 1.5/1.5 group (mean 9.0, p<0.01 v the other two homozygote groups) followed by the 1.3/1.3 group (mean 7.2, NS v the 1.1/1.1 group) and the 1.1/1.1 group (mean 4.0). The SAA/CRP ratio was significantly higher in patients receiving corticosteroids regardless of the presence of allele 1.5. No clear differences in the ratio between patients with or without amyloidosis were found. CONCLUSION—The SAA1.5 allele and corticosteroid treatment had a positive influence on SAA concentrations in serum. These findings are important when evaluating SAA concentration in inflammatory diseases and when considering the cause or treatment of amyloidosis.
Full Text
The Full Text of this article is available as a PDF (144.8 KB).
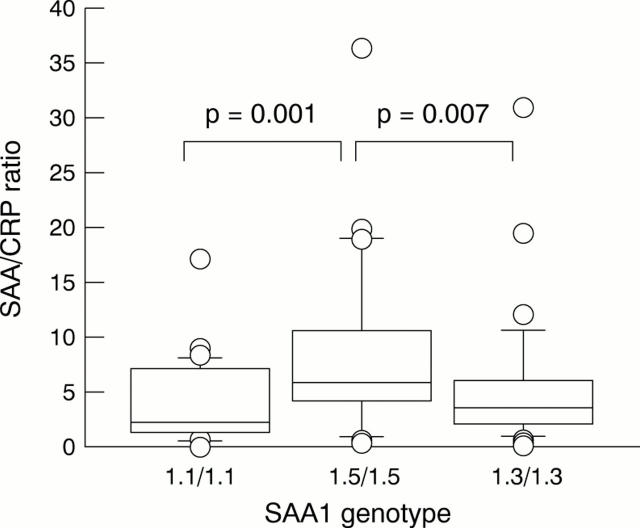
Figure 1 .
Box plots of serum amyloid A/C reactive protein (SAA/CRP) ratios of the three SAA1 homozygote groups. Boxes indicate 25th to 75th centiles; lines in boxes, median values; and upper and lower bars, range from 0 to 100th centile. Subjects with 1.5/1.5 had a higher SAA/CRP ratio than the other two groups. No significant differences were noted between subjects with 1.1/1.1 and 1.3/1.3 genotypes.
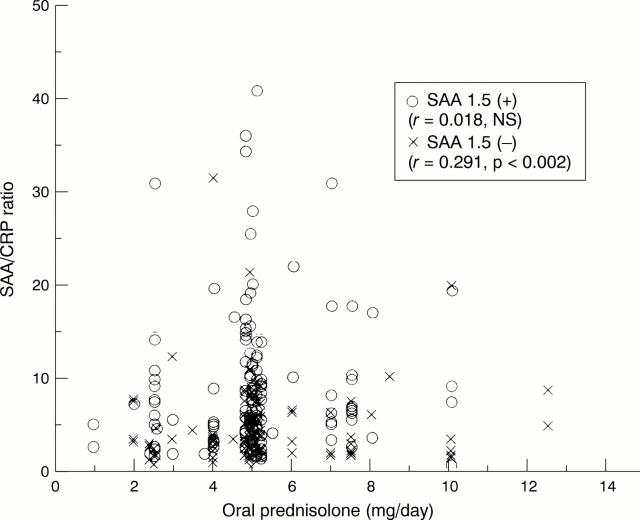
Figure 2 .
Relation between the serum amyloid A/C reactive protein (SAA/CRP) ratio and dose of prednisolone. Significant correlation was observed in subjects with the allele SAA1.5 (n=111), but not in those without it (n=138). Data from five subjects were not included in the plot because of isolated data.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnett F. C., Edworthy S. M., Bloch D. A., McShane D. J., Fries J. F., Cooper N. S., Healey L. A., Kaplan S. R., Liang M. H., Luthra H. S. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum. 1988 Mar;31(3):315–324. doi: 10.1002/art.1780310302. [DOI] [PubMed] [Google Scholar]
- Baba S., Masago S. A., Takahashi T., Kasama T., Sugimura H., Tsugane S., Tsutsui Y., Shirasawa H. A novel allelic variant of serum amyloid A, SAA1 gamma: genomic evidence, evolution, frequency, and implication as a risk factor for reactive systemic AA-amyloidosis. Hum Mol Genet. 1995 Jun;4(6):1083–1087. doi: 10.1093/hmg/4.6.1083. [DOI] [PubMed] [Google Scholar]
- Baumann H., Gauldie J. The acute phase response. Immunol Today. 1994 Feb;15(2):74–80. doi: 10.1016/0167-5699(94)90137-6. [DOI] [PubMed] [Google Scholar]
- Betts J. C., Edbrooke M. R., Thakker R. V., Woo P. The human acute-phase serum amyloid A gene family: structure, evolution and expression in hepatoma cells. Scand J Immunol. 1991 Oct;34(4):471–482. doi: 10.1111/j.1365-3083.1991.tb01570.x. [DOI] [PubMed] [Google Scholar]
- Booth D. R., Booth S. E., Gillmore J. D., Hawkins P. N., Pepys M. B. SAA1 alleles as risk factors in reactive systemic AA amyloidosis. Amyloid. 1998 Dec;5(4):262–265. doi: 10.3109/13506129809007299. [DOI] [PubMed] [Google Scholar]
- Cunnane G., Grehan S., Geoghegan S., McCormack C., Shields D., Whitehead A. S., Bresnihan B., Fitzgerald O. Serum amyloid A in the assessment of early inflammatory arthritis. J Rheumatol. 2000 Jan;27(1):58–63. [PubMed] [Google Scholar]
- Dwulet F. E., Wallace D. K., Benson M. D. Amino acid structures of multiple forms of amyloid-related serum protein SAA from a single individual. Biochemistry. 1988 Mar 8;27(5):1677–1682. doi: 10.1021/bi00405a044. [DOI] [PubMed] [Google Scholar]
- Kobayashi H., Tada S., Fuchigami T., Okuda Y., Takasugi K., Matsumoto T., Iida M., Aoyagi K., Iwashita A., Daimaru Y. Secondary amyloidosis in patients with rheumatoid arthritis: diagnostic and prognostic value of gastroduodenal biopsy. Br J Rheumatol. 1996 Jan;35(1):44–49. doi: 10.1093/rheumatology/35.1.44. [DOI] [PubMed] [Google Scholar]
- Kushner I. The acute phase response: an overview. Methods Enzymol. 1988;163:373–383. doi: 10.1016/0076-6879(88)63037-0. [DOI] [PubMed] [Google Scholar]
- Laakso M., Mutru O., Isomäki H., Koota K. Mortality from amyloidosis and renal diseases in patients with rheumatoid arthritis. Ann Rheum Dis. 1986 Aug;45(8):663–667. doi: 10.1136/ard.45.8.663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liepnieks J. J., Kluve-Beckerman B., Benson M. D. Characterization of amyloid A protein in human secondary amyloidosis: the predominant deposition of serum amyloid A1. Biochim Biophys Acta. 1995 Jan 25;1270(1):81–86. doi: 10.1016/0925-4439(94)00076-3. [DOI] [PubMed] [Google Scholar]
- Lozanski G., Jiang S. L., Samols D., Kushner I. C-reactive protein and serum amyloid A mRNA stability following induction by cytokines. Cytokine. 1996 Jul;8(7):534–540. doi: 10.1006/cyto.1996.0072. [DOI] [PubMed] [Google Scholar]
- Malle E., De Beer F. C. Human serum amyloid A (SAA) protein: a prominent acute-phase reactant for clinical practice. Eur J Clin Invest. 1996 Jun;26(6):427–435. doi: 10.1046/j.1365-2362.1996.159291.x. [DOI] [PubMed] [Google Scholar]
- Maury C. P. Comparative study of serum amyloid A protein and C-reactive protein in disease. Clin Sci (Lond) 1985 Feb;68(2):233–238. doi: 10.1042/cs0680233. [DOI] [PubMed] [Google Scholar]
- Maury C. P., Teppo A. M. Comparative study of serum amyloid-related protein SAA, C-reactive protein, and beta 2-microglobulin as markers of renal allograft rejection. Clin Nephrol. 1984 Dec;22(6):284–292. [PubMed] [Google Scholar]
- Miwata H., Yamada T., Okada M., Kudo T., Kimura H., Morishima T. Serum amyloid A protein in acute viral infections. Arch Dis Child. 1993 Feb;68(2):210–214. doi: 10.1136/adc.68.2.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okuda Y., Takasugi K., Oyama T., Oyama H., Nanba S., Miyamoto T. Intractable diarrhoea associated with secondary amyloidosis in rheumatoid arthritis. Ann Rheum Dis. 1997 Sep;56(9):535–541. doi: 10.1136/ard.56.9.535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okuda Y., Yamada T., Takasugi K., Takeda M., Nanba S., Onishi M., Miyamoto T., Inoue Y. [Serum amyloid A (SAA) 1, SAA 2 and apolipoprotein E isotype frequencies in rheumatoid arthritis patients with AA amyloidosis]. Ryumachi. 1999 Feb;39(1):3–10. [PubMed] [Google Scholar]
- Sipe J. Revised nomenclature for serum amyloid A (SAA). Nomenclature Committee of the International Society of Amyloidosis. Part 2. Amyloid. 1999 Mar;6(1):67–70. doi: 10.3109/13506129908993291. [DOI] [PubMed] [Google Scholar]
- Smith J. W., Colombo J. L., McDonald T. L. Comparison of serum amyloid A and C-reactive protein as indicators of lung inflammation in corticosteroid treated and non-corticosteroid treated cystic fibrosis patients. J Clin Lab Anal. 1992;6(4):219–224. doi: 10.1002/jcla.1860060410. [DOI] [PubMed] [Google Scholar]
- Steel D. M., Whitehead A. S. The major acute phase reactants: C-reactive protein, serum amyloid P component and serum amyloid A protein. Immunol Today. 1994 Feb;15(2):81–88. doi: 10.1016/0167-5699(94)90138-4. [DOI] [PubMed] [Google Scholar]
- Yamada T. Analysis of serum amyloid A1 exon 4 polymorphism in Japanese population. Amyloid. 2000 Jun;7(2):118–120. doi: 10.3109/13506120009146248. [DOI] [PubMed] [Google Scholar]
- Yamada T., Nomata Y., Sugita O., Okada M. A rapid method for measuring serum amyloid A protein by latex agglutination nephelometric immunoassay. Ann Clin Biochem. 1993 Jan;30(Pt 1):72–76. doi: 10.1177/000456329303000112. [DOI] [PubMed] [Google Scholar]
- Yamada T. Serum amyloid A (SAA): a concise review of biology, assay methods and clinical usefulness. Clin Chem Lab Med. 1999 Apr;37(4):381–388. doi: 10.1515/CCLM.1999.063. [DOI] [PubMed] [Google Scholar]
- Yamada T., Wada A., Itoh Y., Itoh K. Serum amyloid A1 alleles and plasma concentrations of serum amyloid A. Amyloid. 1999 Sep;6(3):199–204. doi: 10.3109/13506129909007327. [DOI] [PubMed] [Google Scholar]