Abstract
OBJECTIVE—To determine the prevalence of myositis specific autoantibodies (MSAs) and several myositis associated autoantibodies (MAAs) in a large group of patients with myositis. METHODS—A total of 417 patients with myositis from 11 European countries (198 patients with polymyositis (PM), 181 with dermatomyositis (DM), and 38 with inclusion body myositis (IBM)) were serologically analysed by immunoblot, enzyme linked immunosorbent assay (ELISA) and/or immunoprecipitation. RESULTS—Autoantibodies were found in 232 sera (56%), including 157 samples (38%) which contained MSAs. The most commonly detected MSA was anti-Jo-1 (18%). Other anti-synthetase, anti-Mi-2, and anti-SRP autoantibodies were found in 3%, 14%, and 5% of the sera, respectively. A relatively high number of anti-Mi-2 positive PM sera were found (9% of PM sera). The most commonly detected MAA was anti-Ro52 (25%). Anti-PM/Scl-100, anti-PM/Scl-75, anti-Mas, anti-Ro60, anti-La, and anti-U1 snRNP autoantibodies were present in 6%, 3%, 2%, 4%, 5%, and 6% of the sera, respectively. Remarkable associations were noticed between anti-Ro52 and anti-Jo-1 autoantibodies and, in a few sera, also between anti-Jo-1 and anti-SRP or anti-Mi-2 autoantibodies. CONCLUSIONS—The incidence of most of the tested autoantibody activities in this large group of European patients is in agreement with similar studies of Japanese and American patients. The relatively high number of PM sera with anti-Mi-2 reactivity may be explained by the use of multiple recombinant fragments spanning the complete antigen. Furthermore, our data show that some sera may contain more than one type of MSA and confirm the strong association of anti-Ro52 with anti-Jo-1 reactivity.
Full Text
The Full Text of this article is available as a PDF (169.6 KB).
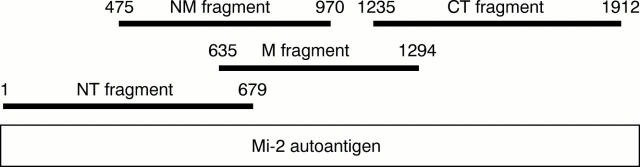
Figure 1 .
Schematic representation of the Mi-2β autoantigen and the fragments used in ELISA. NT = N-terminal; M = middle; NM = N-terminal/middle; CT = C-terminal.
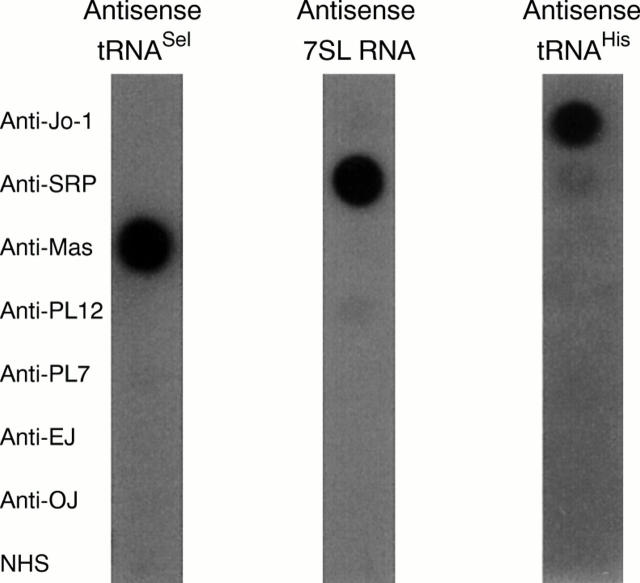
Figure 2 .
Analysis of reference myositis sera and a pool of 10 normal human sera (NHS) by dotblot. RNA precipitated by autoantibodies in myositis sera was spotted on nylon filters and probed with antisense RNA probes to detect anti-Mas, anti-SRP, and anti-Jo-1 reactivity.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alderuccio F., Chan E. K., Tan E. M. Molecular characterization of an autoantigen of PM-Scl in the polymyositis/scleroderma overlap syndrome: a unique and complete human cDNA encoding an apparent 75-kD acidic protein of the nucleolar complex. J Exp Med. 1991 Apr 1;173(4):941–952. doi: 10.1084/jem.173.4.941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allmang C., Petfalski E., Podtelejnikov A., Mann M., Tollervey D., Mitchell P. The yeast exosome and human PM-Scl are related complexes of 3' --> 5' exonucleases. Genes Dev. 1999 Aug 15;13(16):2148–2158. doi: 10.1101/gad.13.16.2148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnett F. C., Targoff I. N., Mimori T., Goldstein R., Warner N. B., Reveille J. D. Interrelationship of major histocompatibility complex class II alleles and autoantibodies in four ethnic groups with various forms of myositis. Arthritis Rheum. 1996 Sep;39(9):1507–1518. doi: 10.1002/art.1780390910. [DOI] [PubMed] [Google Scholar]
- Askanas V., Engel W. K. Sporadic inclusion-body myositis and hereditary inclusion-body myopathies: current concepts of diagnosis and pathogenesis. Curr Opin Rheumatol. 1998 Nov;10(6):530–542. doi: 10.1097/00002281-199811000-00005. [DOI] [PubMed] [Google Scholar]
- Ben-Chetrit E., Fox R. I., Tan E. M. Dissociation of immune responses to the SS-A (Ro) 52-kd and 60-kd polypeptides in systemic lupus erythematosus and Sjögren's syndrome. Arthritis Rheum. 1990 Mar;33(3):349–355. doi: 10.1002/art.1780330307. [DOI] [PubMed] [Google Scholar]
- Benbassat J., Gefel D., Larholt K., Sukenik S., Morgenstern V., Zlotnick A. Prognostic factors in polymyositis/dermatomyositis. A computer-assisted analysis of ninety-two cases. Arthritis Rheum. 1985 Mar;28(3):249–255. doi: 10.1002/art.1780280303. [DOI] [PubMed] [Google Scholar]
- Blüthner M., Bautz F. A. Cloning and characterization of the cDNA coding for a polymyositis-scleroderma overlap syndrome-related nucleolar 100-kD protein. J Exp Med. 1992 Oct 1;176(4):973–980. doi: 10.1084/jem.176.4.973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohan A., Peter J. B. Polymyositis and dermatomyositis (first of two parts). N Engl J Med. 1975 Feb 13;292(7):344–347. doi: 10.1056/NEJM197502132920706. [DOI] [PubMed] [Google Scholar]
- Brouwer R., Vree Egberts W., Jongen P. H., van Engelen B. G., van Venrooij W. J. Frequent occurrence of anti-tRNA(His) autoantibodies that recognize a conformational epitope in sera of patients with myositis. Arthritis Rheum. 1998 Aug;41(8):1428–1437. doi: 10.1002/1529-0131(199808)41:8<1428::AID-ART12>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- Bunn C. C., Bernstein R. M., Mathews M. B. Autoantibodies against alanyl-tRNA synthetase and tRNAAla coexist and are associated with myositis. J Exp Med. 1986 May 1;163(5):1281–1291. doi: 10.1084/jem.163.5.1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalakas M. C. Polymyositis, dermatomyositis and inclusion-body myositis. N Engl J Med. 1991 Nov 21;325(21):1487–1498. doi: 10.1056/NEJM199111213252107. [DOI] [PubMed] [Google Scholar]
- Frank M. B., McCubbin V., Trieu E., Wu Y., Isenberg D. A., Targoff I. N. The association of anti-Ro52 autoantibodies with myositis and scleroderma autoantibodies. J Autoimmun. 1999 Mar;12(2):137–142. doi: 10.1006/jaut.1998.0265. [DOI] [PubMed] [Google Scholar]
- Garlepp M. J. Genetics of the idiopathic inflammatory myopathies. Curr Opin Rheumatol. 1996 Nov;8(6):514–520. doi: 10.1097/00002281-199611000-00004. [DOI] [PubMed] [Google Scholar]
- Ge Q., Frank M. B., O'Brien C., Targoff I. N. Cloning of a complementary DNA coding for the 100-kD antigenic protein of the PM-Scl autoantigen. J Clin Invest. 1992 Aug;90(2):559–570. doi: 10.1172/JCI115895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ge Q., Nilasena D. S., O'Brien C. A., Frank M. B., Targoff I. N. Molecular analysis of a major antigenic region of the 240-kD protein of Mi-2 autoantigen. J Clin Invest. 1995 Oct;96(4):1730–1737. doi: 10.1172/JCI118218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ge Q., Wu Y., Trieu E. P., Targoff I. N. Analysis of the specificity of anti-PM-Scl autoantibodies. Arthritis Rheum. 1994 Oct;37(10):1445–1452. doi: 10.1002/art.1780371007. [DOI] [PubMed] [Google Scholar]
- Gelpi C., Sontheimer E. J., Rodriguez-Sanchez J. L. Autoantibodies against a serine tRNA-protein complex implicated in cotranslational selenocysteine insertion. Proc Natl Acad Sci U S A. 1992 Oct 15;89(20):9739–9743. doi: 10.1073/pnas.89.20.9739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hausmanowa-Petrusewicz I., Kowalska-Oledzka E., Miller F. W., Jarzabek-Chorzelska M., Targoff I. N., Blaszczyk-Kostanecka M., Jablonska S. Clinical, serologic, and immunogenetic features in Polish patients with idiopathic inflammatory myopathies. Arthritis Rheum. 1997 Jul;40(7):1257–1266. doi: 10.1002/1529-0131(199707)40:7<1257::AID-ART10>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- Hengstman G. J., van Engelen B. G., Badrising U. A., van den Hoogen F. H., van Venrooij W. J. Presence of the anti-Jo-1 autoantibody excludes inclusion body myositis. Ann Neurol. 1998 Sep;44(3):423–423. doi: 10.1002/ana.410440325. [DOI] [PubMed] [Google Scholar]
- Hengstman G. J., van Venrooij W. J., Vencovsky J., Moutsopoulos H. M., van Engelen B. G. The relative prevalence of dermatomyositis and polymyositis in Europe exhibits a latitudinal gradient. Ann Rheum Dis. 2000 Feb;59(2):141–142. doi: 10.1136/ard.59.2.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirakata M., Mimori T., Akizuki M., Craft J., Hardin J. A., Homma M. Autoantibodies to small nuclear and cytoplasmic ribonucleoproteins in Japanese patients with inflammatory muscle disease. Arthritis Rheum. 1992 Apr;35(4):449–456. doi: 10.1002/art.1780350415. [DOI] [PubMed] [Google Scholar]
- Hirakata M., Suwa A., Nagai S., Kron M. A., Trieu E. P., Mimori T., Akizuki M., Targoff I. N. Anti-KS: identification of autoantibodies to asparaginyl-transfer RNA synthetase associated with interstitial lung disease. J Immunol. 1999 Feb 15;162(4):2315–2320. [PubMed] [Google Scholar]
- Hochberg M. C., Feldman D., Stevens M. B. Adult onset polymyositis/dermatomyositis: an analysis of clinical and laboratory features and survival in 76 patients with a review of the literature. Semin Arthritis Rheum. 1986 Feb;15(3):168–178. doi: 10.1016/0049-0172(86)90014-4. [DOI] [PubMed] [Google Scholar]
- Holden D. J., Brownell A. K., Fritzler M. J. Clinical and serologic features of patients with polymyositis or dermatomyositis. Can Med Assoc J. 1985 Mar 15;132(6):649–653. [PMC free article] [PubMed] [Google Scholar]
- Kalovidouris A. E. Immune aspects of myositis. Curr Opin Rheumatol. 1992 Dec;4(6):809–814. [PubMed] [Google Scholar]
- Klein Gunnewiek J. M., van de Putte L. B., van Venrooij W. J. The U1 snRNP complex: an autoantigen in connective tissue diseases. An update. Clin Exp Rheumatol. 1997 Sep-Oct;15(5):549–560. [PubMed] [Google Scholar]
- Koh E. T., Seow A., Ong B., Ratnagopal P., Tjia H., Chng H. H. Adult onset polymyositis/dermatomyositis: clinical and laboratory features and treatment response in 75 patients. Ann Rheum Dis. 1993 Dec;52(12):857–861. doi: 10.1136/ard.52.12.857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leff R. L., Burgess S. H., Miller F. W., Love L. A., Targoff I. N., Dalakas M. C., Joffe M. M., Plotz P. H. Distinct seasonal patterns in the onset of adult idiopathic inflammatory myopathy in patients with anti-Jo-1 and anti-signal recognition particle autoantibodies. Arthritis Rheum. 1991 Nov;34(11):1391–1396. doi: 10.1002/art.1780341108. [DOI] [PubMed] [Google Scholar]
- Love L. A., Leff R. L., Fraser D. D., Targoff I. N., Dalakas M., Plotz P. H., Miller F. W. A new approach to the classification of idiopathic inflammatory myopathy: myositis-specific autoantibodies define useful homogeneous patient groups. Medicine (Baltimore) 1991 Nov;70(6):360–374. doi: 10.1097/00005792-199111000-00002. [DOI] [PubMed] [Google Scholar]
- Low S. C., Berry M. J. Knowing when not to stop: selenocysteine incorporation in eukaryotes. Trends Biochem Sci. 1996 Jun;21(6):203–208. [PubMed] [Google Scholar]
- Lundberg I., Nennesmo I., Hedfors E. A clinical, serological, and histopathological study of myositis patients with and without anti-RNP antibodies. Semin Arthritis Rheum. 1992 Oct;22(2):127–138. doi: 10.1016/0049-0172(92)90006-y. [DOI] [PubMed] [Google Scholar]
- Lütcke H., Dobberstein B. Structure and function of signal recognition particle (SRP). Mol Biol Rep. 1993 Aug;18(2):143–147. doi: 10.1007/BF00986769. [DOI] [PubMed] [Google Scholar]
- Miller F. W. Humoral immunity and immunogenetics in the idiopathic inflammatory myopathies. Curr Opin Rheumatol. 1991 Dec;3(6):902–910. doi: 10.1097/00002281-199112000-00002. [DOI] [PubMed] [Google Scholar]
- Oddis C. V., Okano Y., Rudert W. A., Trucco M., Duquesnoy R. J., Medsger T. A., Jr Serum autoantibody to the nucleolar antigen PM-Scl. Clinical and immunogenetic associations. Arthritis Rheum. 1992 Oct;35(10):1211–1217. doi: 10.1002/art.1780351014. [DOI] [PubMed] [Google Scholar]
- Okada N., Mimori T., Mukai R., Kashiwagi H., Hardin J. A. Characterization of human autoantibodies that selectively precipitate the 7SL RNA component of the signal recognition particle. J Immunol. 1987 May 15;138(10):3219–3223. [PubMed] [Google Scholar]
- Pruijn G. J., Simons F. H., van Venrooij W. J. Intracellular localization and nucleocytoplasmic transport of Ro RNP components. Eur J Cell Biol. 1997 Oct;74(2):123–132. [PubMed] [Google Scholar]
- Reichlin M., Arnett F. C., Jr Multiplicity of antibodies in myositis sera. Arthritis Rheum. 1984 Oct;27(10):1150–1156. doi: 10.1002/art.1780271011. [DOI] [PubMed] [Google Scholar]
- Roux S., Seelig H. P., Meyer O. Significance of Mi-2 autoantibodies in polymyositis and dermatomyositis. J Rheumatol. 1998 Feb;25(2):395–396. [PubMed] [Google Scholar]
- Rutjes S. A., Vree Egberts W. T., Jongen P., Van Den Hoogen F., Pruijn G. J., Van Venrooij W. J. Anti-Ro52 antibodies frequently co-occur with anti-Jo-1 antibodies in sera from patients with idiopathic inflammatory myopathy. Clin Exp Immunol. 1997 Jul;109(1):32–40. doi: 10.1046/j.1365-2249.1997.4081308.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schellekens G. A., Visser H., de Jong B. A., van den Hoogen F. H., Hazes J. M., Breedveld F. C., van Venrooij W. J. The diagnostic properties of rheumatoid arthritis antibodies recognizing a cyclic citrullinated peptide. Arthritis Rheum. 2000 Jan;43(1):155–163. doi: 10.1002/1529-0131(200001)43:1<155::AID-ANR20>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- Seelig H. P., Moosbrugger I., Ehrfeld H., Fink T., Renz M., Genth E. The major dermatomyositis-specific Mi-2 autoantigen is a presumed helicase involved in transcriptional activation. Arthritis Rheum. 1995 Oct;38(10):1389–1399. doi: 10.1002/art.1780381006. [DOI] [PubMed] [Google Scholar]
- Targoff I. N. Immune manifestations of inflammatory muscle disease. Rheum Dis Clin North Am. 1994 Nov;20(4):857–880. [PubMed] [Google Scholar]
- Targoff I. N., Johnson A. E., Miller F. W. Antibody to signal recognition particle in polymyositis. Arthritis Rheum. 1990 Sep;33(9):1361–1370. doi: 10.1002/art.1780330908. [DOI] [PubMed] [Google Scholar]
- Targoff I. N., Reichlin M. The association between Mi-2 antibodies and dermatomyositis. Arthritis Rheum. 1985 Jul;28(7):796–803. doi: 10.1002/art.1780280711. [DOI] [PubMed] [Google Scholar]
- Tymms K. E., Webb J. Dermatopolymyositis and other connective tissue diseases: a review of 105 cases. J Rheumatol. 1985 Dec;12(6):1140–1148. [PubMed] [Google Scholar]
- Uthman I., Vázquez-Abad D., Senécal J. L. Distinctive features of idiopathic inflammatory myopathies in French Canadians. Semin Arthritis Rheum. 1996 Aug;26(1):447–458. doi: 10.1016/s0049-0172(96)80025-4. [DOI] [PubMed] [Google Scholar]
- Wade P. A., Gegonne A., Jones P. L., Ballestar E., Aubry F., Wolffe A. P. Mi-2 complex couples DNA methylation to chromatin remodelling and histone deacetylation. Nat Genet. 1999 Sep;23(1):62–66. doi: 10.1038/12664. [DOI] [PubMed] [Google Scholar]
- Woodage T., Basrai M. A., Baxevanis A. D., Hieter P., Collins F. S. Characterization of the CHD family of proteins. Proc Natl Acad Sci U S A. 1997 Oct 14;94(21):11472–11477. doi: 10.1073/pnas.94.21.11472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y., Ng H. H., Erdjument-Bromage H., Tempst P., Bird A., Reinberg D. Analysis of the NuRD subunits reveals a histone deacetylase core complex and a connection with DNA methylation. Genes Dev. 1999 Aug 1;13(15):1924–1935. doi: 10.1101/gad.13.15.1924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Mühlen C. A., Tan E. M. Autoantibodies in the diagnosis of systemic rheumatic diseases. Semin Arthritis Rheum. 1995 Apr;24(5):323–358. doi: 10.1016/s0049-0172(95)80004-2. [DOI] [PubMed] [Google Scholar]