Abstract
OBJECTIVE—To determine if a new inhibitor, esculetin (EST), can block resorption of cartilage. METHODS—Interleukin 1α (IL1α, 0.04-5 ng/ml) and oncostatin M (OSM, 0.4-50 ng/ml) were used to stimulate the release of proteoglycan and collagen from bovine nasal cartilage and human articular cartilage in explant culture. Proteoglycan and collagen loss were assessed by dimethylmethylene blue and hydroxyproline assays, respectively. Collagenase levels were measured by assay of bioactivity and by enzyme linked immunosorbent assay (ELISA). The effects of EST on the expression of matrix metalloproteinases (MMPs) and tissue inhibitor of metalloproteinase-1 (TIMP-1) in the transformed human chondrocyte cell line T/C28a4 were assessed by northern blot analysis. TIMP-1 protein levels were assayed by ELISA. The effect of EST on the MMP-1 promoter was assessed using a promoter-luciferase construct in transient transfection studies. RESULTS—EST inhibited proteoglycan and collagen resorption in a dose dependent manner with significant decreases seen at 66 µM and 100 µM EST, respectively. Collagenolytic activity was significantly decreased in bovine nasal cartilage cultures. In human articular cartilage, EST also inhibited IL1α + OSM stimulated resorption and decreased MMP-1 levels. TIMP-1 levels were not altered compared with controls. In T/C28a4 chondrocytes the IL1α + OSM induced expression of MMP-1, MMP-3, and MMP-13 mRNA was reduced to control levels by 250 µM EST. TIMP-1 mRNA levels were unaffected by EST treatment. All cytokine stimulation of an MMP-1 luciferase-promoter construct was lost in the presence of the inhibitor. CONCLUSION—EST inhibits degradation of bovine nasal cartilage and human articular cartilage stimulated to resorb with IL1α + OSM.
Full Text
The Full Text of this article is available as a PDF (185.3 KB).
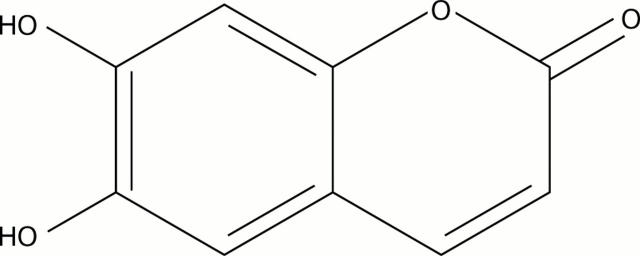
Figure 1 .
Esculetin (6,7-dihydroxycoumarin).
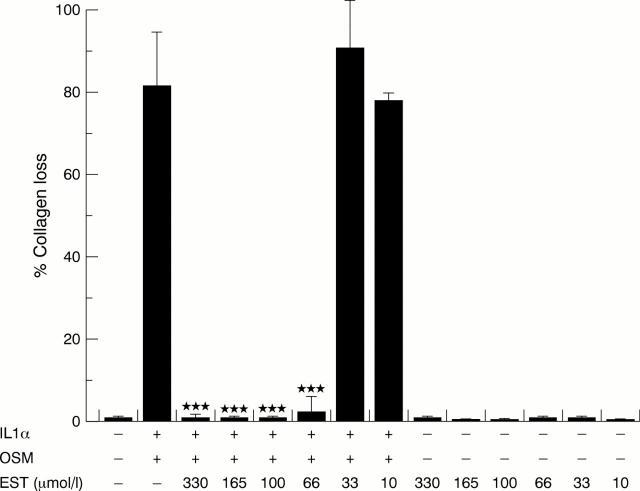
Figure 2 .
Esculetin (EST) inhibits collagen loss from bovine nasal cartilage stimulated to resorb with interleukin 1α (IL1α) and oncostatin M (OSM). Bovine nasal cartilage discs were cultured for 14 days with IL1α (1 ng/ml) in combination with OSM (10 ng/ml) ± EST (10-330 µmol/l). Media were harvested and replenished at day 7. Collagen release is expressed as a percentage of the total present in the explant. The cumulative collagen loss over 14 days of culture is presented. Results are expressed as mean (SD) (n=4). Statistical analyses compared collagen loss seen with IL1α + OSM alone to that seen with IL1α + OSM + EST. ***p<0.001.
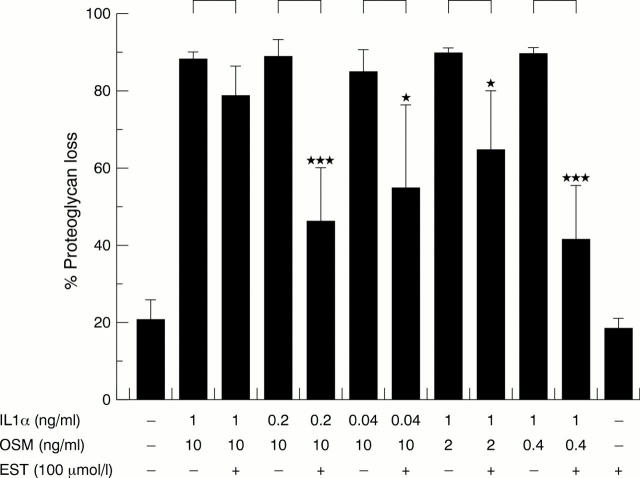
Figure 3 .
Esculetin (EST) inhibits proteoglycan loss from bovine nasal cartilage. Bovine nasal cartilage discs were incubated for 14 days with interleukin 1α (IL1α) and oncostatin M (OSM) at the concentrations indicated ± EST (100 µmol/l). Media were harvested and replenished at day 7; media and cartilage were harvested at day 14. Proteoglycan loss is expressed as a percentage of the total present in each explant; the data illustrated are for proteoglycan loss by day 7 of culture. Results are mean (SD) (n=4). Statistical analyses compared proteoglycan loss seen with IL1α + OSM treatment to that seen with IL1α + OSM + EST for each set of cytokine concentrations as indicated by the bars. ***p<0.001, *p<0.05.
Figure 4 .
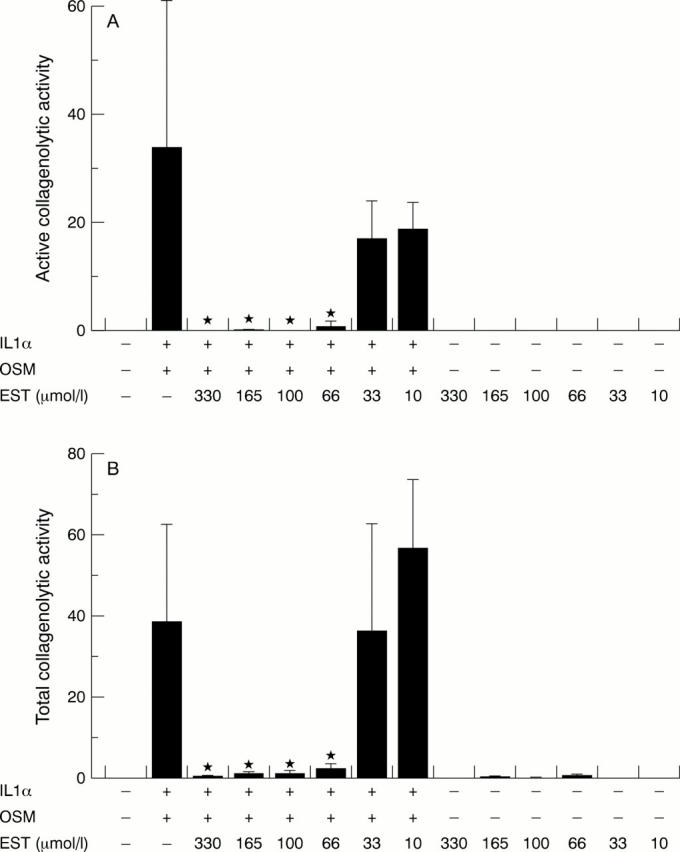
Esculetin (EST) inhibits interleukin 1α (IL1α) + oncostatin M (OSM) induced collagenolytic activity in bovine nasal cartilage. Bovine nasal cartilage discs were incubated for 14 days with IL1α (1 ng/ml) in combination with OSM (10 ng/ml) ± EST (10-330 µmol/l), with media changes at day 7. Media were harvested at day 14 and assessed by using the diffuse collagen fibril assay for active collagenolytic activity (A) and total collagenolytic activity by the inclusion of p-aminophenylmercuric acetate (0.7 mmol/l) in the assay (B). Statistical analyses compared the activity of IL1α + OSM treatment with that of IL1α + OSM + EST treatment (n=4). *p<0.05.
Figure 5 .
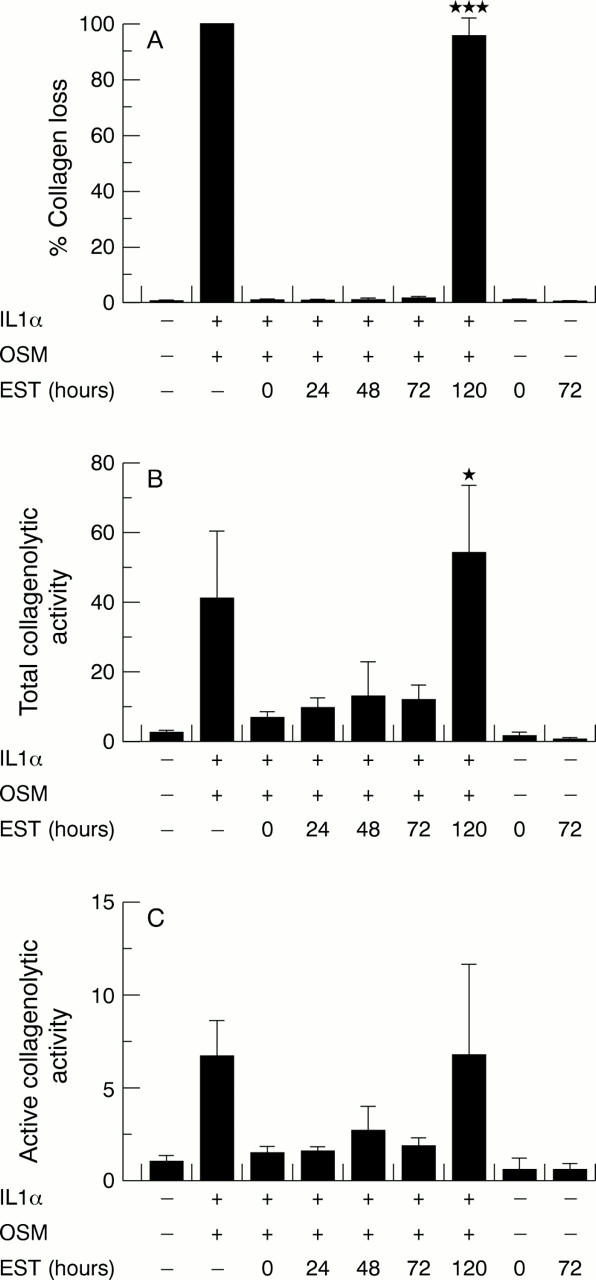
Esculetin (EST) inhibits collagen loss and collagenolytic activity up to 72 hours after cytokine stimulation of bovine nasal cartilage. Bovine nasal cartilage discs were cultured for 14 days with interleukin 1α (IL1α; 1 ng/ml) and oncostatin M (OSM; 10 ng/ml). EST (100 µmol/l) was added simultaneously with the cytokines or up to 120 hours thereafter. Media were harvested and replenished at day 7; media and cartilage were harvested at day 14. (A) Cumulative collagen loss over 14 days of culture. Collagenolytic activity was measured in day 7 media in the presence (B) or absence (C) of the metalloproteinase activator, p-aminophenylmercuric acetate (0.7 mmol/l). Statistical analyses of collagen loss and collagenolytic activity levels compared IL1α + OSM + EST (0 hours) with IL1α + OSM + EST added at time points thereafter (n=4). ***p<0.001, *p<0.05.
Figure 6 .
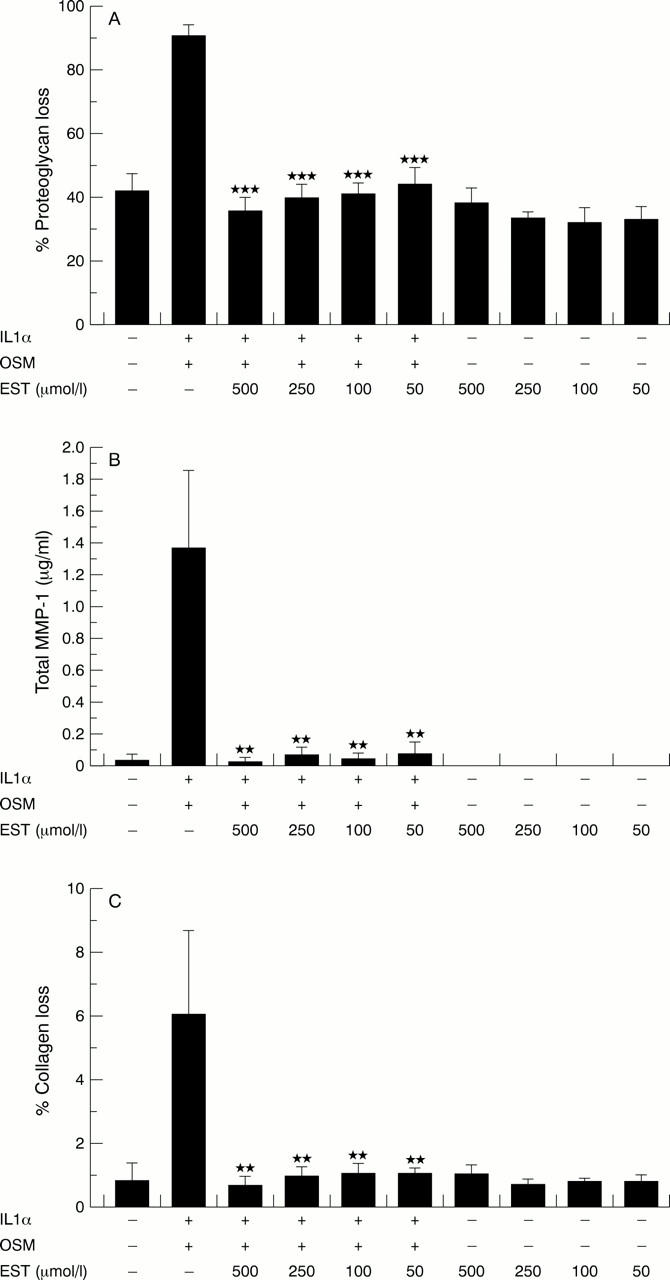
Esculetin (EST) inhibits interleukin 1α (IL1α) + oncostatin M (OSM) stimulated resorption of human articular cartilage. Pieces of human articular cartilage (2 mm3) were cultured with IL1α (5 ng/ml) in combination with OSM (50 ng/ml) ± EST (50-500 µmol/l). Media were harvested and replenished at days 7 and 14; media and cartilage were harvested at day 21. Proteoglycan and collagen release are presented as a percentage of the total. (A) Cumulative proteoglycan loss over 21 days of culture. (B) Cumulative MMP-1 levels over 21 days of culture. (C) Cumulative collagen loss over 21 days of culture. Statistical analyses compared the data for IL1α + OSM with that for the IL1α + OSM + EST treatments (n=4). ***p<0.001, **p<0.01.
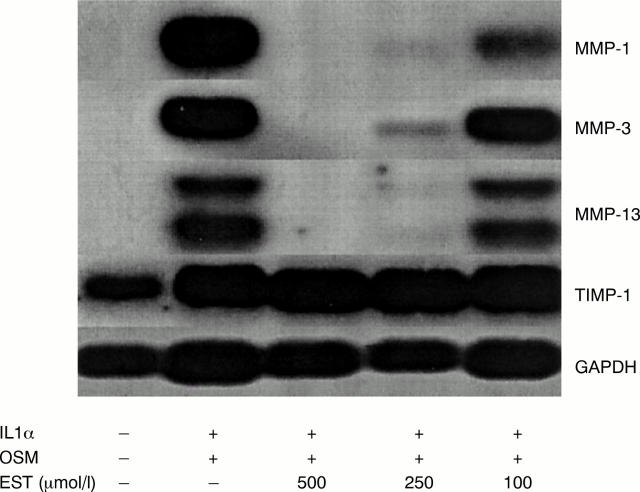
Figure 7 .
Esculetin (EST) reduces the interleukin 1α (IL1α) + oncostatin M (OSM) induced expression of MMP-1, MMP-3, and MMP-13 mRNA in T/C28a4 chondrocytes. T/C28a4 chondrocytes were stimulated for 24 hours with IL1α (1 ng/ml) in combination with OSM (10 ng/ml) ± EST (100-500 µmol/l). Total RNA was harvested, northern blotted, and the membranes probed for MMP-1, MMP-3, MMP-13, and TIMP-1 mRNA. Glyceraldehyde-3-phosphate dehydrogenase was used to correct for RNA loading. The data are representative of three separate experiments.
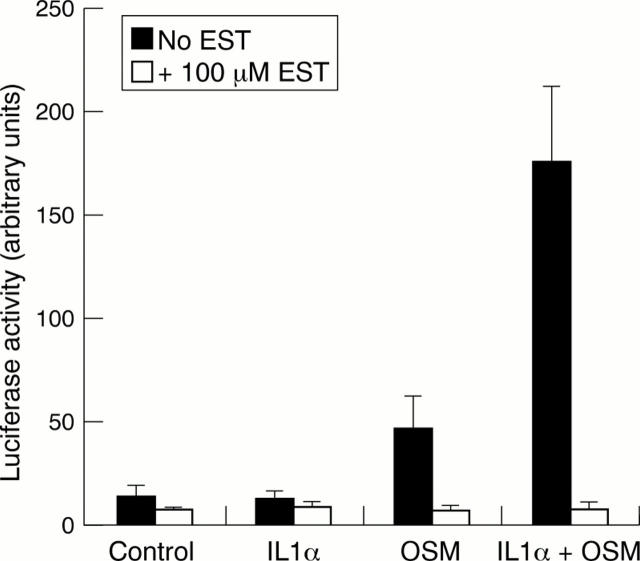
Figure 8 .
Esculetin (EST) inhibits matrix metalloproteinase-1 (MMP-1) promoter activity. T/C28a4 cells were transiently transfected with an MMP-1/luciferase promoter construct. Two days after transfection, cells were incubated with interleukin 1α (IL1α; 1 ng/ml) and/or OSM (10 ng/ml) ± EST (100 µmol/l) for 24 hours. Cells were then harvested and luciferase activities measured, and normalised to the amount of DNA plasmid in each well. The results represent the mean (SD) (n=3) of a representative experiment performed three times.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arner E. C., Hughes C. E., Decicco C. P., Caterson B., Tortorella M. D. Cytokine-induced cartilage proteoglycan degradation is mediated by aggrecanase. Osteoarthritis Cartilage. 1998 May;6(3):214–228. doi: 10.1053/joca.1998.0114. [DOI] [PubMed] [Google Scholar]
- Arner E. C., Pratta M. A., Decicco C. P., Xue C. B., Newton R. C., Trzaskos J. M., Magolda R. L., Tortorella M. D. Aggrecanase. A target for the design of inhibitors of cartilage degradation. Ann N Y Acad Sci. 1999 Jun 30;878:92–107. doi: 10.1111/j.1749-6632.1999.tb07676.x. [DOI] [PubMed] [Google Scholar]
- Arner E. C., Pratta M. A., Trzaskos J. M., Decicco C. P., Tortorella M. D. Generation and characterization of aggrecanase. A soluble, cartilage-derived aggrecan-degrading activity. J Biol Chem. 1999 Mar 5;274(10):6594–6601. doi: 10.1074/jbc.274.10.6594. [DOI] [PubMed] [Google Scholar]
- Barrett A. J. Alpha 2-macroglobulin. Methods Enzymol. 1981;80(Pt 100):737–754. doi: 10.1016/s0076-6879(81)80056-0. [DOI] [PubMed] [Google Scholar]
- Benbow U., Brinckerhoff C. E. The AP-1 site and MMP gene regulation: what is all the fuss about? Matrix Biol. 1997 Mar;15(8-9):519–526. doi: 10.1016/s0945-053x(97)90026-3. [DOI] [PubMed] [Google Scholar]
- Billington C. J., Clark I. M., Cawston T. E. An aggrecan-degrading activity associated with chondrocyte membranes. Biochem J. 1998 Nov 15;336(Pt 1):207–212. doi: 10.1042/bj3360207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bottomley K. M., Borkakoti N., Bradshaw D., Brown P. A., Broadhurst M. J., Budd J. M., Elliott L., Eyers P., Hallam T. J., Handa B. K. Inhibition of bovine nasal cartilage degradation by selective matrix metalloproteinase inhibitors. Biochem J. 1997 Apr 15;323(Pt 2):483–488. doi: 10.1042/bj3230483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brewster M., Lewis E. J., Wilson K. L., Greenham A. K., Bottomley K. M. Ro 32-3555, an orally active collagenase selective inhibitor, prevents structural damage in the STR/ORT mouse model of osteoarthritis. Arthritis Rheum. 1998 Sep;41(9):1639–1644. doi: 10.1002/1529-0131(199809)41:9<1639::AID-ART15>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- Brinckerhoff C. E. Joint destruction in arthritis: metalloproteinases in the spotlight. Arthritis Rheum. 1991 Sep;34(9):1073–1075. doi: 10.1002/art.1780340902. [DOI] [PubMed] [Google Scholar]
- Cawston T. E., Barrett A. J. A rapid and reproducible assay for collagenase using [1-14C]acetylated collagen. Anal Biochem. 1979 Nov 1;99(2):340–345. doi: 10.1016/s0003-2697(79)80017-2. [DOI] [PubMed] [Google Scholar]
- Cawston T. E., Curry V. A., Summers C. A., Clark I. M., Riley G. P., Life P. F., Spaull J. R., Goldring M. B., Koshy P. J., Rowan A. D. The role of oncostatin M in animal and human connective tissue collagen turnover and its localization within the rheumatoid joint. Arthritis Rheum. 1998 Oct;41(10):1760–1771. doi: 10.1002/1529-0131(199810)41:10<1760::AID-ART8>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- Cawston T. E., Ellis A. J., Humm G., Lean E., Ward D., Curry V. Interleukin-1 and oncostatin M in combination promote the release of collagen fragments from bovine nasal cartilage in culture. Biochem Biophys Res Commun. 1995 Oct 4;215(1):377–385. doi: 10.1006/bbrc.1995.2476. [DOI] [PubMed] [Google Scholar]
- Cawston T. E. Metalloproteinase inhibitors and the prevention of connective tissue breakdown. Pharmacol Ther. 1996;70(3):163–182. doi: 10.1016/0163-7258(96)00015-0. [DOI] [PubMed] [Google Scholar]
- Cawston T., Billington C., Cleaver C., Elliott S., Hui W., Koshy P., Shingleton B., Rowan A. The regulation of MMPs and TIMPs in cartilage turnover. Ann N Y Acad Sci. 1999 Jun 30;878:120–129. doi: 10.1111/j.1749-6632.1999.tb07678.x. [DOI] [PubMed] [Google Scholar]
- Clark I. M., Powell L. K., Wright J. K., Cawston T. E., Hazleman B. L. Monoclonal antibodies against human fibroblast collagenase and the design of an enzyme-linked immunosorbent assay to measure total collagenase. Matrix. 1992 Dec;12(6):475–480. doi: 10.1016/s0934-8832(11)80092-2. [DOI] [PubMed] [Google Scholar]
- Conaghan P. G., Brooks P. Disease-modifying antirheumatic drugs, including methotrexate, gold, antimalarials, and D-penicillamine. Curr Opin Rheumatol. 1995 May;7(3):167–173. doi: 10.1097/00002281-199505000-00003. [DOI] [PubMed] [Google Scholar]
- DiMartino M., Wolff C., High W., Stroup G., Hoffman S., Laydon J., Lee J. C., Bertolini D., Galloway W. A., Crimmin M. J. Anti-arthritic activity of hydroxamic acid-based pseudopeptide inhibitors of matrix metalloproteinases and TNF alpha processing. Inflamm Res. 1997 Jun;46(6):211–215. doi: 10.1007/s000110050175. [DOI] [PubMed] [Google Scholar]
- Dingle J. T., Page Thomas D. P., King B., Bard D. R. In vivo studies of articular tissue damage mediated by catabolin/interleukin 1. Ann Rheum Dis. 1987 Jul;46(7):527–533. doi: 10.1136/ard.46.7.527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodge G. R., Pidoux I., Poole A. R. The degradation of type II collagen in rheumatoid arthritis: an immunoelectron microscopic study. Matrix. 1991 Nov;11(5):330–338. doi: 10.1016/s0934-8832(11)80204-0. [DOI] [PubMed] [Google Scholar]
- Ellis A. J., Curry V. A., Powell E. K., Cawston T. E. The prevention of collagen breakdown in bovine nasal cartilage by TIMP, TIMP-2 and a low molecular weight synthetic inhibitor. Biochem Biophys Res Commun. 1994 May 30;201(1):94–101. doi: 10.1006/bbrc.1994.1673. [DOI] [PubMed] [Google Scholar]
- Flannery C. R., Lark M. W., Sandy J. D. Identification of a stromelysin cleavage site within the interglobular domain of human aggrecan. Evidence for proteolysis at this site in vivo in human articular cartilage. J Biol Chem. 1992 Jan 15;267(2):1008–1014. [PubMed] [Google Scholar]
- Fort P., Marty L., Piechaczyk M., el Sabrouty S., Dani C., Jeanteur P., Blanchard J. M. Various rat adult tissues express only one major mRNA species from the glyceraldehyde-3-phosphate-dehydrogenase multigenic family. Nucleic Acids Res. 1985 Mar 11;13(5):1431–1442. doi: 10.1093/nar/13.5.1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fosang A. J., Last K., Knäuper V., Neame P. J., Murphy G., Hardingham T. E., Tschesche H., Hamilton J. A. Fibroblast and neutrophil collagenases cleave at two sites in the cartilage aggrecan interglobular domain. Biochem J. 1993 Oct 1;295(Pt 1):273–276. doi: 10.1042/bj2950273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freije J. M., Díez-Itza I., Balbín M., Sánchez L. M., Blasco R., Tolivia J., López-Otín C. Molecular cloning and expression of collagenase-3, a novel human matrix metalloproteinase produced by breast carcinomas. J Biol Chem. 1994 Jun 17;269(24):16766–16773. [PubMed] [Google Scholar]
- Goldring M. B., Birkhead J. R., Suen L. F., Yamin R., Mizuno S., Glowacki J., Arbiser J. L., Apperley J. F. Interleukin-1 beta-modulated gene expression in immortalized human chondrocytes. J Clin Invest. 1994 Dec;94(6):2307–2316. doi: 10.1172/JCI117595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenwald R. A., Golub L. M., Ramamurthy N. S., Chowdhury M., Moak S. A., Sorsa T. In vitro sensitivity of the three mammalian collagenases to tetracycline inhibition: relationship to bone and cartilage degradation. Bone. 1998 Jan;22(1):33–38. doi: 10.1016/s8756-3282(97)00221-4. [DOI] [PubMed] [Google Scholar]
- Hascall V. C., Handley C. J., McQuillan D. J., Hascall G. K., Robinson H. C., Lowther D. A. The effect of serum on biosynthesis of proteoglycans by bovine articular cartilage in culture. Arch Biochem Biophys. 1983 Jul 1;224(1):206–223. doi: 10.1016/0003-9861(83)90205-9. [DOI] [PubMed] [Google Scholar]
- Hasty K. A., Jeffrey J. J., Hibbs M. S., Welgus H. G. The collagen substrate specificity of human neutrophil collagenase. J Biol Chem. 1987 Jul 25;262(21):10048–10052. [PubMed] [Google Scholar]
- Henriet P., Blavier L., Declerck Y. A. Tissue inhibitors of metalloproteinases (TIMP) in invasion and proliferation. APMIS. 1999 Jan;107(1):111–119. doi: 10.1111/j.1699-0463.1999.tb01533.x. [DOI] [PubMed] [Google Scholar]
- Hirt B. Selective extraction of polyoma DNA from infected mouse cell cultures. J Mol Biol. 1967 Jun 14;26(2):365–369. doi: 10.1016/0022-2836(67)90307-5. [DOI] [PubMed] [Google Scholar]
- Jubb R. W., Fell H. B. The breakdown of collagen by chondrocytes. J Pathol. 1980 Mar;130(3):159–167. doi: 10.1002/path.1711300304. [DOI] [PubMed] [Google Scholar]
- Koshy P. J., Rowan A. D., Life P. F., Cawston T. E. 96-Well plate assays for measuring collagenase activity using (3)H-acetylated collagen. Anal Biochem. 1999 Nov 15;275(2):202–207. doi: 10.1006/abio.1999.4310. [DOI] [PubMed] [Google Scholar]
- Krane S. M. Clinical importance of metalloproteinases and their inhibitors. Ann N Y Acad Sci. 1994 Sep 6;732:1–10. doi: 10.1111/j.1749-6632.1994.tb24719.x. [DOI] [PubMed] [Google Scholar]
- Kuettner K. E. Biochemistry of articular cartilage in health and disease. Clin Biochem. 1992 Jun;25(3):155–163. doi: 10.1016/0009-9120(92)90224-g. [DOI] [PubMed] [Google Scholar]
- Lark M. W., Gordy J. T., Weidner J. R., Ayala J., Kimura J. H., Williams H. R., Mumford R. A., Flannery C. R., Carlson S. S., Iwata M. Cell-mediated catabolism of aggrecan. Evidence that cleavage at the "aggrecanase" site (Glu373-Ala374) is a primary event in proteolysis of the interglobular domain. J Biol Chem. 1995 Feb 10;270(6):2550–2556. doi: 10.1074/jbc.270.6.2550. [DOI] [PubMed] [Google Scholar]
- Lewis E. J., Bishop J., Bottomley K. M., Bradshaw D., Brewster M., Broadhurst M. J., Brown P. A., Budd J. M., Elliott L., Greenham A. K. Ro 32-3555, an orally active collagenase inhibitor, prevents cartilage breakdown in vitro and in vivo. Br J Pharmacol. 1997 Jun;121(3):540–546. doi: 10.1038/sj.bjp.0701150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luyten F. P., Hascall V. C., Nissley S. P., Morales T. I., Reddi A. H. Insulin-like growth factors maintain steady-state metabolism of proteoglycans in bovine articular cartilage explants. Arch Biochem Biophys. 1988 Dec;267(2):416–425. doi: 10.1016/0003-9861(88)90047-1. [DOI] [PubMed] [Google Scholar]
- Martel-Pelletier J., McCollum R., Fujimoto N., Obata K., Cloutier J. M., Pelletier J. P. Excess of metalloproteases over tissue inhibitor of metalloprotease may contribute to cartilage degradation in osteoarthritis and rheumatoid arthritis. Lab Invest. 1994 Jun;70(6):807–815. [PubMed] [Google Scholar]
- Martel-Pelletier J., Mineau F., Tardif G., Fernandes J. C., Ranger P., Loose L., Pelletier J. P. Tenidap reduces the level of interleukin 1 receptors and collagenase expression in human arthritic synovial fibroblasts. J Rheumatol. 1996 Jan;23(1):24–31. [PubMed] [Google Scholar]
- McCachren S. S. Expression of metalloproteinases and metalloproteinase inhibitor in human arthritic synovium. Arthritis Rheum. 1991 Sep;34(9):1085–1093. doi: 10.1002/art.1780340904. [DOI] [PubMed] [Google Scholar]
- Mort J. S., Dodge G. R., Roughley P. J., Liu J., Finch S. J., DiPasquale G., Poole A. R. Direct evidence for active metalloproteinases mediating matrix degradation in interleukin 1-stimulated human articular cartilage. Matrix. 1993 Mar;13(2):95–102. doi: 10.1016/s0934-8832(11)80068-5. [DOI] [PubMed] [Google Scholar]
- Murphy G., Cockett M. I., Stephens P. E., Smith B. J., Docherty A. J. Stromelysin is an activator of procollagenase. A study with natural and recombinant enzymes. Biochem J. 1987 Nov 15;248(1):265–268. doi: 10.1042/bj2480265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagase H., Woessner J. F., Jr Matrix metalloproteinases. J Biol Chem. 1999 Jul 30;274(31):21491–21494. doi: 10.1074/jbc.274.31.21491. [DOI] [PubMed] [Google Scholar]
- Page Thomas D. P., King B., Stephens T., Dingle J. T. In vivo studies of cartilage regeneration after damage induced by catabolin/interleukin-1. Ann Rheum Dis. 1991 Feb;50(2):75–80. doi: 10.1136/ard.50.2.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelletier J. P., Mineau F., Faure M. P., Martel-Pelletier J. Imbalance between the mechanisms of activation and inhibition of metalloproteases in the early lesions of experimental osteoarthritis. Arthritis Rheum. 1990 Oct;33(10):1466–1476. doi: 10.1002/art.1780331003. [DOI] [PubMed] [Google Scholar]
- Plumpton T. A., Clark I. M., Plumpton C., Calvin J., Cawston T. E. Development of an enzyme-linked immunosorbent assay to measure total TIMP-1 (free TIMP-1 and TIMP-1 in combination with matrix-metalloproteinases) and measurement of TIMP 1 and CRP in serum. Clin Chim Acta. 1995 Sep 15;240(2):137–154. doi: 10.1016/0009-8981(95)06137-7. [DOI] [PubMed] [Google Scholar]
- Rutter J. L., Benbow U., Coon C. I., Brinckerhoff C. E. Cell-type specific regulation of human interstitial collagenase-1 gene expression by interleukin-1 beta (IL-1 beta) in human fibroblasts and BC-8701 breast cancer cells. J Cell Biochem. 1997 Sep 1;66(3):322–336. [PubMed] [Google Scholar]
- Sah R. L., Chen A. C., Grodzinsky A. J., Trippel S. B. Differential effects of bFGF and IGF-I on matrix metabolism in calf and adult bovine cartilage explants. Arch Biochem Biophys. 1994 Jan;308(1):137–147. doi: 10.1006/abbi.1994.1020. [DOI] [PubMed] [Google Scholar]
- Sandy J. D., Boynton R. E., Flannery C. R. Analysis of the catabolism of aggrecan in cartilage explants by quantitation of peptides from the three globular domains. J Biol Chem. 1991 May 5;266(13):8198–8205. [PubMed] [Google Scholar]
- Steinmeyer J., Daufeldt S. Pharmacological influence of antirheumatic drugs on proteoglycans from interleukin-1 treated articular cartilage. Biochem Pharmacol. 1997 Jun 1;53(11):1627–1635. doi: 10.1016/s0006-2952(97)00066-x. [DOI] [PubMed] [Google Scholar]
- Stricklin G. P., Bauer E. A., Jeffrey J. J., Eisen A. Z. Human skin collagenase: isolation of precursor and active forms from both fibroblast and organ cultures. Biochemistry. 1977 Apr 19;16(8):1607–1615. doi: 10.1021/bi00627a013. [DOI] [PubMed] [Google Scholar]
- Suzuki K., Enghild J. J., Morodomi T., Salvesen G., Nagase H. Mechanisms of activation of tissue procollagenase by matrix metalloproteinase 3 (stromelysin). Biochemistry. 1990 Nov 6;29(44):10261–10270. doi: 10.1021/bi00496a016. [DOI] [PubMed] [Google Scholar]
- Tyler J. A. Articular cartilage cultured with catabolin (pig interleukin 1) synthesizes a decreased number of normal proteoglycan molecules. Biochem J. 1985 May 1;227(3):869–878. doi: 10.1042/bj2270869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyler J. A. Insulin-like growth factor 1 can decrease degradation and promote synthesis of proteoglycan in cartilage exposed to cytokines. Biochem J. 1989 Jun 1;260(2):543–548. doi: 10.1042/bj2600543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincenti M. P., Clark I. M., Brinckerhoff C. E. Using inhibitors of metalloproteinases to treat arthritis. Easier said than done? Arthritis Rheum. 1994 Aug;37(8):1115–1126. doi: 10.1002/art.1780370802. [DOI] [PubMed] [Google Scholar]
- Watanabe K., Ito A., Sato T., Saito T., Hayashi H., Niitani Y. Esculetin suppresses proteoglycan metabolism by inhibiting the production of matrix metalloproteinases in rabbit chondrocytes. Eur J Pharmacol. 1999 Apr 16;370(3):297–305. doi: 10.1016/s0014-2999(99)00143-0. [DOI] [PubMed] [Google Scholar]
- Woessner J. F., Jr The family of matrix metalloproteinases. Ann N Y Acad Sci. 1994 Sep 6;732:11–21. doi: 10.1111/j.1749-6632.1994.tb24720.x. [DOI] [PubMed] [Google Scholar]
- Yamada H., Kikuchi T., Nemoto O., Obata K., Sato H., Seiki M., Shinmei M. Effects of indomethacin on the production of matrix metalloproteinase-3 and tissue inhibitor of metalloproteinases-1 by human articular chondrocytes. J Rheumatol. 1996 Oct;23(10):1739–1743. [PubMed] [Google Scholar]