Abstract
OBJECTIVES—Recent evidence suggests that prolactin (PRL) plays a part in the pathogenesis of systemic lupus erythematosus (SLE). Because B cell hyperreactivity and autoantibodies are characteristic hallmarks of SLE, this study aimed at assessing the impact of this pituitary hormone on IgG production by stimulating peripheral blood mononuclear cells (PBMC) with PRL. METHODS—PBMC from 11 patients with SLE assessed by the ECLAM score and eight healthy controls were incubated with PRL and cultured for seven days. IgG production was measured by enzyme linked immunosorbent assay (ELISA). RESULTS—Spontaneous IgG production of SLE PBMC was significantly enhanced compared with that found in healthy controls. After PRL stimulation, the IgG concentrations of supernatants from SLE PBMC were significantly higher than those of unstimulated PBMC (median 394 ng/ml). Of note, the physiological concentration of PRL (20 ng/ml) induced IgG production more effectively (median 1139 ng/ml) than PRL at 100 ng/ml (median 1029 ng/ml). In contrast, preincubation with PRL did not stimulate IgG production in normal PBMC. A significant correlation between PRL induced IgG production and the disease activity (ECLAM) of the patients with SLE was seen. Moreover, the maximum amount of PRL induced IgG depended on the serum PRL concentrations of the patients with SLE. CONCLUSIONS—The results suggest that PBMC from patients with SLE have an extraordinarily high susceptibility to PRL, showing the most striking effect at a concentration usually found in vivo. This indicates a potential role for mild hyperprolactinaemia in the pathogenesis of SLE, influencing both IgG production and disease activity.
Full Text
The Full Text of this article is available as a PDF (136.4 KB).
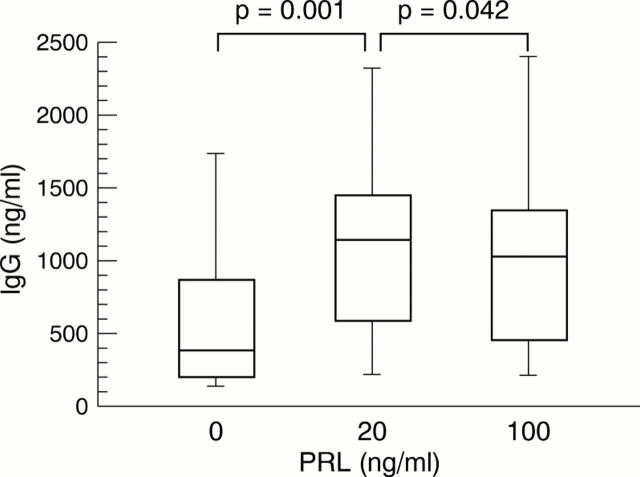
Figure 1 .
Concentration of total IgG in the supernatants of peripheral blood mononuclear cells (PBMC) obtained from 11 patients with systemic lupus erythematosus (SLE) after incubation with prolactin (PRL) at concentrations of 20 and 100 ng/ml, respectively, and without PRL (0) for seven days. Significant differences were seen between the IgG production by PBMC from patients with SLE after and without stimulation with PRL (20 and 100 ng/ml, respectively) (Wilcoxon test and Bonferroni correction, p=0.043).
Figure 2 .
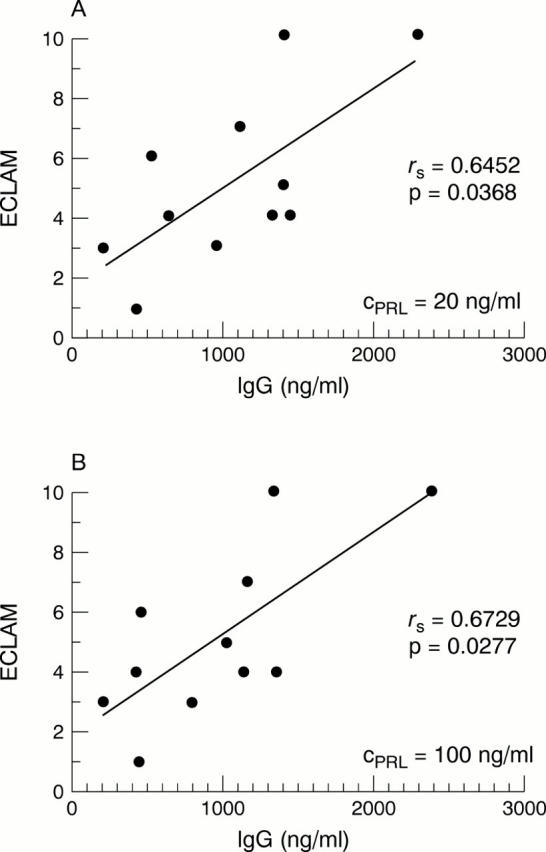
Correlation of the IgG concentration of the supernatants of peripheral blood mononuclear cells from patients with systemic lupus erythematosus (SLE) after incubation with prolactin (PRL) at two concentrations (CPRL = 20 ng/ml and 100 ng/ml) and the ECLAM score of the patients with SLE analysed.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alvarez-Nemegyei J., Cobarrubias-Cobos A., Escalante-Triay F., Sosa-Muñoz J., Miranda J. M., Jara L. J. Bromocriptine in systemic lupus erythematosus: a double-blind, randomized, placebo-controlled study. Lupus. 1998;7(6):414–419. doi: 10.1191/096120398678920334. [DOI] [PubMed] [Google Scholar]
- Ben-Jonathan N., Mershon J. L., Allen D. L., Steinmetz R. W. Extrapituitary prolactin: distribution, regulation, functions, and clinical aspects. Endocr Rev. 1996 Dec;17(6):639–669. doi: 10.1210/edrv-17-6-639. [DOI] [PubMed] [Google Scholar]
- Berczi I., Nagy E., Asa S. L., Kovacs K. Pituitary hormones and contact sensitivity in rats. Allergy. 1983 Jul;38(5):325–330. doi: 10.1111/j.1398-9995.1983.tb04126.x. [DOI] [PubMed] [Google Scholar]
- Buskila D., Berezin M., Gur H., Lin H. C., Alosachie I., Terryberry J. W., Barka N., Shen B., Peter J. B., Shoenfeld Y. Autoantibody profile in the sera of women with hyperprolactinemia. J Autoimmun. 1995 Jun;8(3):415–424. doi: 10.1006/jaut.1995.0033. [DOI] [PubMed] [Google Scholar]
- Buskila D., Lorber M., Neumann L., Flusser D., Shoenfeld Y. No correlation between prolactin levels and clinical activity in patients with systemic lupus erythematosus. J Rheumatol. 1996 Apr;23(4):629–632. [PubMed] [Google Scholar]
- Böyum A. Isolation of mononuclear cells and granulocytes from human blood. Isolation of monuclear cells by one centrifugation, and of granulocytes by combining centrifugation and sedimentation at 1 g. Scand J Clin Lab Invest Suppl. 1968;97:77–89. [PubMed] [Google Scholar]
- Elbourne K. B., Keisler D., McMurray R. W. Differential effects of estrogen and prolactin on autoimmune disease in the NZB/NZW F1 mouse model of systemic lupus erythematosus. Lupus. 1998;7(6):420–427. doi: 10.1191/096120398678920352. [DOI] [PubMed] [Google Scholar]
- Ferrari C., Boghen M., Paracchi A., Rampini P., Raiteri F., Benco R., Romussi M., Codecasa F., Mucci M., Bianco M. Thyroid autoimmunity in hyperprolactinaemic disorders. Acta Endocrinol (Copenh) 1983 Sep;104(1):35–41. doi: 10.1530/acta.0.1040035. [DOI] [PubMed] [Google Scholar]
- Fletcher-Chiappini S. E., Compton M. M., LaVoie H. A., Day E. B., Witorsch R. J., Comptom M. M. Glucocorticoid-prolactin interactions in Nb2 lymphoma cells: antiproliferative versus anticytolytic effects. Proc Soc Exp Biol Med. 1993 Mar;202(3):345–352. doi: 10.3181/00379727-202-43545. [DOI] [PubMed] [Google Scholar]
- Fuh G., Cunningham B. C., Fukunaga R., Nagata S., Goeddel D. V., Wells J. A. Rational design of potent antagonists to the human growth hormone receptor. Science. 1992 Jun 19;256(5064):1677–1680. doi: 10.1126/science.256.5064.1677. [DOI] [PubMed] [Google Scholar]
- Gutiérrez M. A., Anaya J. M., Scopelitis E., Citera G., Silveira L., Espinoza L. R. Hyperprolactinaemia in primary Sjögren's syndrome. Ann Rheum Dis. 1994 Jun;53(6):425–425. doi: 10.1136/ard.53.6.425-a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutiérrez M. A., Molina J. F., Jara L. J., Cuéllar M. L., García C., Gutiérrez-Ureña S., Gharavi A., Espinoza L. R. Prolactin and systemic lupus erythematosus: prolactin secretion by SLE lymphocytes and proliferative (autocrine) activity. Lupus. 1995 Oct;4(5):348–352. doi: 10.1177/096120339500400504. [DOI] [PubMed] [Google Scholar]
- Gutiérrez M. A., Molina J. F., Jara L. J., García C., Gutiérrez-Ureña S., Cuéllar M. L., Gharavi A., Espinoza L. R. Prolactin-induced immunoglobulin and autoantibody production by peripheral blood mononuclear cells from systemic lupus erythematosus and normal individuals. Int Arch Allergy Immunol. 1996 Mar;109(3):229–235. doi: 10.1159/000237242. [DOI] [PubMed] [Google Scholar]
- Hedner L. P., Bynke G. Endogenous iridocyclitis relieved during treatment with bromocriptine. Am J Ophthalmol. 1985 Oct 15;100(4):618–619. doi: 10.1016/0002-9394(85)90697-x. [DOI] [PubMed] [Google Scholar]
- Honegger J., Fahlbusch R., Bornemann A., Hensen J., Buchfelder M., Müller M., Nomikos P. Lymphocytic and granulomatous hypophysitis: experience with nine cases. Neurosurgery. 1997 Apr;40(4):713–723. doi: 10.1097/00006123-199704000-00010. [DOI] [PubMed] [Google Scholar]
- Huang C. M., Chou C. T. Hyperprolactinemia in systemic lupus erythematosus. Zhonghua Yi Xue Za Zhi (Taipei) 1997 Jan;59(1):37–41. [PubMed] [Google Scholar]
- Jackson R. D., Wortsman J., Malarkey W. B. Characterization of a large molecular weight prolactin in women with idiopathic hyperprolactinemia and normal menses. J Clin Endocrinol Metab. 1985 Aug;61(2):258–264. doi: 10.1210/jcem-61-2-258. [DOI] [PubMed] [Google Scholar]
- Jara L. J., Gomez-Sanchez C., Silveira L. H., Martinez-Osuna P., Vasey F. B., Espinoza L. R. Hyperprolactinemia in systemic lupus erythematosus: association with disease activity. Am J Med Sci. 1992 Apr;303(4):222–226. doi: 10.1097/00000441-199204000-00003. [DOI] [PubMed] [Google Scholar]
- Jimena P., Aguirre M. A., López-Curbelo A., de Andrés M., Garcia-Courtay C., Cuadrado M. J. Prolactin levels in patients with systemic lupus erythematosus: a case controlled study. Lupus. 1998;7(6):383–386. doi: 10.1191/096120398678920361. [DOI] [PubMed] [Google Scholar]
- Krause I., Blumenfeld Z., Malchinsky M., Cohen S., Blank M., Eldor A., Weksler B., Schweitzer K., Shoenfeld Y. Anti-endothelial cell antibodies in the sera of hyperprolactinemic women. Lupus. 1998;7(6):377–382. doi: 10.1191/096120398678920316. [DOI] [PubMed] [Google Scholar]
- Kucharz E. J., Jarczyk R., Jonderko G., Rubisz-Brezezińska J., Brzezińska-Wcislo L. High serum level of prolactin in patients with systemic sclerosis. Clin Rheumatol. 1996 May;15(3):314–314. doi: 10.1007/BF02229718. [DOI] [PubMed] [Google Scholar]
- Lahat N., Miller A., Shtiller R., Touby E. Differential effects of prolactin upon activation and differentiation of human B lymphocytes. J Neuroimmunol. 1993 Aug;47(1):35–40. doi: 10.1016/0165-5728(93)90282-4. [DOI] [PubMed] [Google Scholar]
- Lavalle C., Loyo E., Paniagua R., Bermudez J. A., Herrera J., Graef A., Gonzalez-Barcena D., Fraga A. Correlation study between prolactin and androgens in male patients with systemic lupus erythematosus. J Rheumatol. 1987 Apr;14(2):268–272. [PubMed] [Google Scholar]
- Leaños A., Pascoe D., Fraga A., Blanco-Favela F. Anti-prolactin autoantibodies in systemic lupus erythematosus patients with associated hyperprolactinemia. Lupus. 1998;7(6):398–403. doi: 10.1191/096120398678920280. [DOI] [PubMed] [Google Scholar]
- Leff M. A., Buckley D. J., Krumenacker J. S., Reed J. C., Miyashita T., Buckley A. R. Rapid modulation of the apoptosis regulatory genes, bcl-2 and bax by prolactin in rat Nb2 lymphoma cells. Endocrinology. 1996 Dec;137(12):5456–5462. doi: 10.1210/endo.137.12.8940371. [DOI] [PubMed] [Google Scholar]
- Lever E. G., McKerron C. G. Auto-immune Addison's disease associated with hyperprolactinaemia. Clin Endocrinol (Oxf) 1984 Oct;21(4):451–457. doi: 10.1111/j.1365-2265.1984.tb03231.x. [DOI] [PubMed] [Google Scholar]
- McMurray R. W., Weidensaul D., Allen S. H., Walker S. E. Efficacy of bromocriptine in an open label therapeutic trial for systemic lupus erythematosus. J Rheumatol. 1995 Nov;22(11):2084–2091. [PubMed] [Google Scholar]
- McMurray R., Keisler D., Kanuckel K., Izui S., Walker S. E. Prolactin influences autoimmune disease activity in the female B/W mouse. J Immunol. 1991 Dec 1;147(11):3780–3787. [PubMed] [Google Scholar]
- Miranda J. M., Prieto R. E., Paniagua R., Garcia G., Amato D., Barile L., Jara L. J. Clinical significance of serum and urine prolactin levels in lupus glomerulonephritis. Lupus. 1998;7(6):387–391. doi: 10.1191/096120398678920307. [DOI] [PubMed] [Google Scholar]
- Mok C. C., Lau C. S., Tam S. C. Prolactin profile in a cohort of Chinese systemic lupus erythematosus patients. Br J Rheumatol. 1997 Sep;36(9):986–989. doi: 10.1093/rheumatology/36.9.986. [DOI] [PubMed] [Google Scholar]
- Mukherjee P., Mastro A. M., Hymer W. C. Prolactin induction of interleukin-2 receptors on rat splenic lymphocytes. Endocrinology. 1990 Jan;126(1):88–94. doi: 10.1210/endo-126-1-88. [DOI] [PubMed] [Google Scholar]
- Muñoz J. A., Gil A., López-Dupla J. M., Vázquez J. J., González-Gancedo P. Sex hormones in chronic systemic lupus erythematosus. Correlation with clinical and biological parameters. Ann Med Interne (Paris) 1994;145(7):459–463. [PubMed] [Google Scholar]
- Nagy E., Berczi I., Friesen H. G. Regulation of immunity in rats by lactogenic and growth hormones. Acta Endocrinol (Copenh) 1983 Mar;102(3):351–357. doi: 10.1530/acta.0.1020351. [DOI] [PubMed] [Google Scholar]
- Nagy E., Berczi I., Wren G. E., Asa S. L., Kovacs K. Immunomodulation by bromocriptine. Immunopharmacology. 1983 Oct;6(3):231–243. doi: 10.1016/0162-3109(83)90023-1. [DOI] [PubMed] [Google Scholar]
- Neidhart M. Elevated serum prolactin or elevated prolactin/cortisol ratio are associated with autoimmune processes in systemic lupus erythematosus and other connective tissue diseases. J Rheumatol. 1996 Mar;23(3):476–481. [PubMed] [Google Scholar]
- Ostendorf B., Fischer R., Santen R., Schmitz-Linneweber B., Specker C., Schneider M. Hyperprolactinemia in systemic lupus erythematosus? Scand J Rheumatol. 1996;25(2):97–102. doi: 10.3109/03009749609069215. [DOI] [PubMed] [Google Scholar]
- Pauzner R., Urowitz M. B., Gladman D. D., Gough J. M. Prolactin in systemic lupus erythematosus. J Rheumatol. 1994 Nov;21(11):2064–2067. [PubMed] [Google Scholar]
- Picco P., Gattorno M., Buoncompagni A., Facchetti P., Rossi G., Pistoia V. Prolactin and interleukin 6 in prepubertal girls with juvenile chronic arthritis. J Rheumatol. 1998 Feb;25(2):347–351. [PubMed] [Google Scholar]
- Rinkenberger J. L., Wallin J. J., Johnson K. W., Koshland M. E. An interleukin-2 signal relieves BSAP (Pax5)-mediated repression of the immunoglobulin J chain gene. Immunity. 1996 Oct;5(4):377–386. doi: 10.1016/s1074-7613(00)80263-0. [DOI] [PubMed] [Google Scholar]
- Rui H., Lebrun J. J., Kirken R. A., Kelly P. A., Farrar W. L. JAK2 activation and cell proliferation induced by antibody-mediated prolactin receptor dimerization. Endocrinology. 1994 Oct;135(4):1299–1306. doi: 10.1210/endo.135.4.7925093. [DOI] [PubMed] [Google Scholar]
- Tan E. M., Cohen A. S., Fries J. F., Masi A. T., McShane D. J., Rothfield N. F., Schaller J. G., Talal N., Winchester R. J. The 1982 revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum. 1982 Nov;25(11):1271–1277. doi: 10.1002/art.1780251101. [DOI] [PubMed] [Google Scholar]
- Vitali C., Bencivelli W., Isenberg D. A., Smolen J. S., Snaith M. L., Sciuto M., Neri R., Bombardieri S. Disease activity in systemic lupus erythematosus: report of the Consensus Study Group of the European Workshop for Rheumatology Research. II. Identification of the variables indicative of disease activity and their use in the development of an activity score. The European Consensus Study Group for Disease Activity in SLE. Clin Exp Rheumatol. 1992 Sep-Oct;10(5):541–547. [PubMed] [Google Scholar]
- Walker A. M., Montgomery D. W., Saraiya S., Ho T. W., Garewal H. S., Wilson J., Lorand L. Prolactin-immunoglobulin G complexes from human serum act as costimulatory ligands causing proliferation of malignant B lymphocytes. Proc Natl Acad Sci U S A. 1995 Apr 11;92(8):3278–3282. doi: 10.1073/pnas.92.8.3278. [DOI] [PMC free article] [PubMed] [Google Scholar]