Abstract
OBJECTIVES—The significance of the mast cell in the pathogenesis of rheumatic diseases has become more evident. Although mast cell hyperplasia is a feature of rheumatoid arthritis, the nature of mast cell chemoattractants involved in the recruitment of mast cells in joint diseases has not been studied in any detail. In this study the presence of mast cell chemotactic activity in synovial fluids was examined. METHODS—Synovial fluids from seven rheumatoid patients were tested in a modified Boyden chamber, where a human mast cell line was used as responder. The presence of stem cell factor (SCF) and transforming growth factor β (TGFβ) was measured by enzyme linked immunosorbent assay (ELISA). RESULTS—Six of the seven synovial fluids tested exhibited mast cell chemotactic activity. Two characterised human mast cell chemotaxins, SCF and TGFβ, were highly expressed in the synovium. Soluble SCF could be detected in all fluids analysed. Blocking antibodies against SCF or TGFβ almost completely blocked the activity in one fluid, partially blocked the activity in three, and did not affect the activity in two. Treatment of the responder cells with pertussis toxin reduced the migratory response against seven fluids, indicating the presence of chemoattractants mediating their effect through Gi coupled receptors. CONCLUSION—These data demonstrate the presence of multiple factors in synovial fluid acting as mast cell chemoattractants, two of which are SCF and TGFβ that contribute to the effect. These findings may be of importance for developing new strategies to inhibit mast cell accumulation in rheumatic diseases.
Full Text
The Full Text of this article is available as a PDF (213.1 KB).
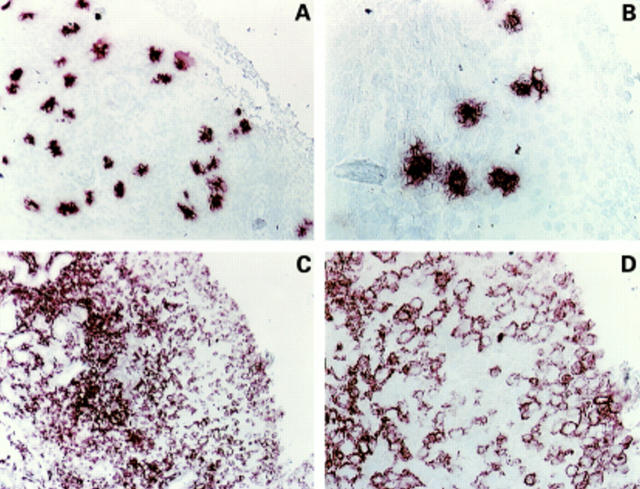
Figure 1 .
Immunolocalisation of mast cells and stem cell factor (SCF) in synovial tissue. Tissues were stained for tryptase (A and B) or SCF (C and D). The figures are from the same patient (RD1), but different sections. The stainings are presented at two different magnifications from the same section. Magnification for A and C is ×80, and for B and D ×200.
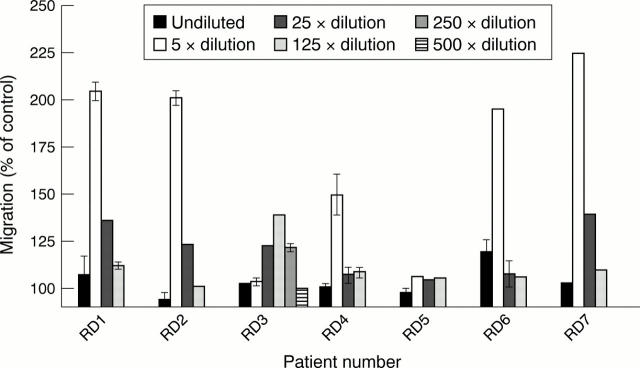
Figure 2 .
Migratory response of human cultured mast cells to synovial fluid. The fluid was diluted in medium and tested for mast cell chemotactic activity. Dilutions: undiluted, 5×, 25×, 125×, 250×, and 500×. Migration was measured after 150 minutes. The results depicted are from three experiments performed in triplicate. Results are given as means (SEM).
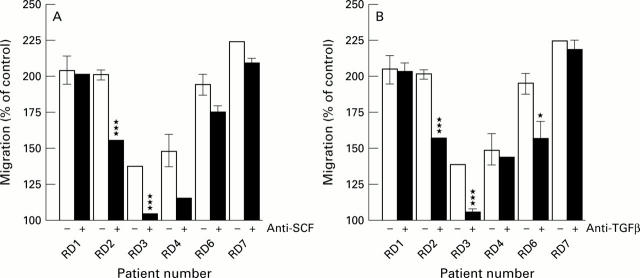
Figure 3 .
Inhibition of activity of synovial fluids in mast cell chemotaxis assay by specific antibodies against (A) SCF (10 µg/ml) and (B) TGFβ (10 µg/ml). Open bars = control, filled bars = with antibody. The results are from three experiments performed in triplicate. Results are given as means (SEM). A significant effect was obtained as indicated: *p<0.05, ***p<0.001.
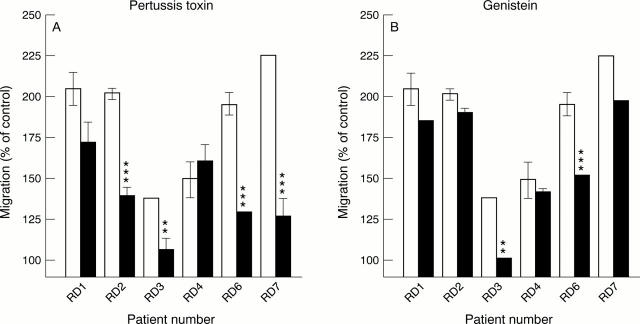
Figure 4 .
Analysis of signal transduction pathways which play a part in mediating mast cell chemoattractant activity in synovial fluids. Mast cells were treated with (A) pertussis toxin, or (B) genistein for 90 minutes before chemotaxis assay. The fluids were used at dilutions giving optimal migration. Open bars = control; filled bars = with inhibitor. The results are from three experiments performed in triplicate. Results are given as means (SEM). A significant effect was obtained as indicated: **p<0.01, ***p<0.001.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen J. B., Manthey C. L., Hand A. R., Ohura K., Ellingsworth L., Wahl S. M. Rapid onset synovial inflammation and hyperplasia induced by transforming growth factor beta. J Exp Med. 1990 Jan 1;171(1):231–247. doi: 10.1084/jem.171.1.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnett F. C., Edworthy S. M., Bloch D. A., McShane D. J., Fries J. F., Cooper N. S., Healey L. A., Kaplan S. R., Liang M. H., Luthra H. S. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum. 1988 Mar;31(3):315–324. doi: 10.1002/art.1780310302. [DOI] [PubMed] [Google Scholar]
- Buckley M. G., Walters C., Wong W. M., Cawley M. I., Ren S., Schwartz L. B., Walls A. F. Mast cell activation in arthritis: detection of alpha- and beta-tryptase, histamine and eosinophil cationic protein in synovial fluid. Clin Sci (Lond) 1997 Oct;93(4):363–370. doi: 10.1042/cs0930363. [DOI] [PubMed] [Google Scholar]
- Butterfield J. H., Weiler D., Dewald G., Gleich G. J. Establishment of an immature mast cell line from a patient with mast cell leukemia. Leuk Res. 1988;12(4):345–355. doi: 10.1016/0145-2126(88)90050-1. [DOI] [PubMed] [Google Scholar]
- CASTOR C. W. The microscopic structure of normal human synovial tissue. Arthritis Rheum. 1960 Apr;3:140–151. doi: 10.1002/art.1780030205. [DOI] [PubMed] [Google Scholar]
- Ceponis A., Konttinen Y. T., Takagi M., Xu J. W., Sorsa T., Matucci-Cerinic M., Santavirta S., Bankl H. C., Valent P. Expression of stem cell factor (SCF) and SCF receptor (c-kit) in synovial membrane in arthritis: correlation with synovial mast cell hyperplasia and inflammation. J Rheumatol. 1998 Dec;25(12):2304–2314. [PubMed] [Google Scholar]
- Crisp A. J., Chapman C. M., Kirkham S. E., Schiller A. L., Krane S. M. Articular mastocytosis in rheumatoid arthritis. Arthritis Rheum. 1984 Aug;27(8):845–851. doi: 10.1002/art.1780270802. [DOI] [PubMed] [Google Scholar]
- Cunnane G., Bjork L., Ulfgren A. K., Lindblad S., FitzGerald O., Bresnihan B., Andersson U. Quantitative analysis of synovial membrane inflammation: a comparison between automated and conventional microscopic measurements. Ann Rheum Dis. 1999 Aug;58(8):493–499. doi: 10.1136/ard.58.8.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldmann M., Brennan F. M., Maini R. N. Role of cytokines in rheumatoid arthritis. Annu Rev Immunol. 1996;14:397–440. doi: 10.1146/annurev.immunol.14.1.397. [DOI] [PubMed] [Google Scholar]
- Frewin D. B., Cleland L. G., Jonsson J. R., Robertson P. W. Histamine levels in human synovial fluid. J Rheumatol. 1986 Feb;13(1):13–14. [PubMed] [Google Scholar]
- Galli S. J., Zsebo K. M., Geissler E. N. The kit ligand, stem cell factor. Adv Immunol. 1994;55:1–96. doi: 10.1016/s0065-2776(08)60508-8. [DOI] [PubMed] [Google Scholar]
- Godfrey H. P., Ilardi C., Engber W., Graziano F. M. Quantitation of human synovial mast cells in rheumatoid arthritis and other rheumatic diseases. Arthritis Rheum. 1984 Aug;27(8):852–856. doi: 10.1002/art.1780270803. [DOI] [PubMed] [Google Scholar]
- Gordon J. R., Burd P. R., Galli S. J. Mast cells as a source of multifunctional cytokines. Immunol Today. 1990 Dec;11(12):458–464. doi: 10.1016/0167-5699(90)90176-a. [DOI] [PubMed] [Google Scholar]
- Gotis-Graham I., Smith M. D., Parker A., McNeil H. P. Synovial mast cell responses during clinical improvement in early rheumatoid arthritis. Ann Rheum Dis. 1998 Nov;57(11):664–671. doi: 10.1136/ard.57.11.664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruber B. L., Marchese M. J., Kew R. R. Transforming growth factor-beta 1 mediates mast cell chemotaxis. J Immunol. 1994 Jun 15;152(12):5860–5867. [PubMed] [Google Scholar]
- Hartmann K., Henz B. M., Krüger-Krasagakes S., Köhl J., Burger R., Guhl S., Haase I., Lippert U., Zuberbier T. C3a and C5a stimulate chemotaxis of human mast cells. Blood. 1997 Apr 15;89(8):2863–2870. [PubMed] [Google Scholar]
- Jose P. J., Moss I. K., Maini R. N., Williams T. J. Measurement of the chemotactic complement fragment C5a in rheumatoid synovial fluids by radioimmunoassay: role of C5a in the acute inflammatory phase. Ann Rheum Dis. 1990 Oct;49(10):747–752. doi: 10.1136/ard.49.10.747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiener H. P., Hofbauer R., Tohidast-Akrad M., Walchshofer S., Redlich K., Bitzan P., Kapiotis S., Steiner G., Smolen J. S., Valent P. Tumor necrosis factor alpha promotes the expression of stem cell factor in synovial fibroblasts and their capacity to induce mast cell chemotaxis. Arthritis Rheum. 2000 Jan;43(1):164–174. doi: 10.1002/1529-0131(200001)43:1<164::AID-ANR21>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- LEVER J. D., FORD E. H. Histological, histochemical and electron microscopic observations on synovial membrane. Anat Rec. 1958 Dec;132(4):525–539. doi: 10.1002/ar.1091320402. [DOI] [PubMed] [Google Scholar]
- Lippert U., Artuc M., Grützkau A., Möller A., Kenderessy-Szabo A., Schadendorf D., Norgauer J., Hartmann K., Schweitzer-Stenner R., Zuberbier T. Expression and functional activity of the IL-8 receptor type CXCR1 and CXCR2 on human mast cells. J Immunol. 1998 Sep 1;161(5):2600–2608. [PubMed] [Google Scholar]
- Malone D. G., Irani A. M., Schwartz L. B., Barrett K. E., Metcalfe D. D. Mast cell numbers and histamine levels in synovial fluids from patients with diverse arthritides. Arthritis Rheum. 1986 Aug;29(8):956–963. doi: 10.1002/art.1780290803. [DOI] [PubMed] [Google Scholar]
- Malone D. G., Wilder R. L., Saavedra-Delgado A. M., Metcalfe D. D. Mast cell numbers in rheumatoid synovial tissues. Correlations with quantitative measures of lymphocytic infiltration and modulation by antiinflammatory therapy. Arthritis Rheum. 1987 Feb;30(2):130–137. doi: 10.1002/art.1780300202. [DOI] [PubMed] [Google Scholar]
- Marone G. Mast cells in rheumatic disorders: mastermind or workhorse? Clin Exp Rheumatol. 1998 May-Jun;16(3):245–249. [PubMed] [Google Scholar]
- Meininger C. J., Yano H., Rottapel R., Bernstein A., Zsebo K. M., Zetter B. R. The c-kit receptor ligand functions as a mast cell chemoattractant. Blood. 1992 Feb 15;79(4):958–963. [PubMed] [Google Scholar]
- Mekori Y. A., Metcalfe D. D. Transforming growth factor-beta prevents stem cell factor-mediated rescue of mast cells from apoptosis after IL-3 deprivation. J Immunol. 1994 Sep 1;153(5):2194–2203. [PubMed] [Google Scholar]
- Metcalfe D. D., Baram D., Mekori Y. A. Mast cells. Physiol Rev. 1997 Oct;77(4):1033–1079. doi: 10.1152/physrev.1997.77.4.1033. [DOI] [PubMed] [Google Scholar]
- Mican J. M., Metcalfe D. D. Arthritis and mast cell activation. J Allergy Clin Immunol. 1990 Oct;86(4 Pt 2):677–683. doi: 10.1016/s0091-6749(05)80240-4. [DOI] [PubMed] [Google Scholar]
- Nilsson G., Blom T., Kusche-Gullberg M., Kjellén L., Butterfield J. H., Sundström C., Nilsson K., Hellman L. Phenotypic characterization of the human mast-cell line HMC-1. Scand J Immunol. 1994 May;39(5):489–498. doi: 10.1111/j.1365-3083.1994.tb03404.x. [DOI] [PubMed] [Google Scholar]
- Nilsson G., Butterfield J. H., Nilsson K., Siegbahn A. Stem cell factor is a chemotactic factor for human mast cells. J Immunol. 1994 Oct 15;153(8):3717–3723. [PubMed] [Google Scholar]
- Nilsson G., Hjertson M., Andersson M., Greiff L., Svensson C., Nilsson K., Siegbahn A. Demonstration of mast-cell chemotactic activity in nasal lavage fluid: characterization of one chemotaxin as c-kit ligand, stem cell factor. Allergy. 1998 Sep;53(9):874–879. doi: 10.1111/j.1398-9995.1998.tb03994.x. [DOI] [PubMed] [Google Scholar]
- Nilsson G., Johnell M., Hammer C. H., Tiffany H. L., Nilsson K., Metcalfe D. D., Siegbahn A., Murphy P. M. C3a and C5a are chemotaxins for human mast cells and act through distinct receptors via a pertussis toxin-sensitive signal transduction pathway. J Immunol. 1996 Aug 15;157(4):1693–1698. [PubMed] [Google Scholar]
- Nilsson G., Metcalfe D. D. Contemporary issues in mast cell biology. Allergy Asthma Proc. 1996 Mar-Apr;17(2):59–63. doi: 10.2500/108854196778645074. [DOI] [PubMed] [Google Scholar]
- Nilsson G., Metcalfe D. D., Taub D. D. Demonstration that platelet-activating factor is capable of activating mast cells and inducing a chemotactic response. Immunology. 2000 Feb;99(2):314–319. doi: 10.1046/j.1365-2567.2000.00972.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilsson G., Mikovits J. A., Metcalfe D. D., Taub D. D. Mast cell migratory response to interleukin-8 is mediated through interaction with chemokine receptor CXCR2/Interleukin-8RB. Blood. 1999 May 1;93(9):2791–2797. [PubMed] [Google Scholar]
- Olsson N., Piek E., ten Dijke P., Nilsson G. Human mast cell migration in response to members of the transforming growth factor-beta family. J Leukoc Biol. 2000 Mar;67(3):350–356. doi: 10.1002/jlb.67.3.350. [DOI] [PubMed] [Google Scholar]
- Olsson N., Rak S., Nilsson G. Demonstration of mast cell chemotactic activity in bronchoalveolar lavage fluid collected from asthmatic patients before and during pollen season. J Allergy Clin Immunol. 2000 Mar;105(3):455–461. doi: 10.1067/mai.2000.104380. [DOI] [PubMed] [Google Scholar]
- Olsson N., Siegbahn A., Nilsson G. Serum amyloid A induces chemotaxis of human mast cells by activating a pertussis toxin-sensitive signal transduction pathway. Biochem Biophys Res Commun. 1999 Jan 8;254(1):143–146. doi: 10.1006/bbrc.1998.9911. [DOI] [PubMed] [Google Scholar]
- Taketazu F., Kato M., Gobl A., Ichijo H., ten Dijke P., Itoh J., Kyogoku M., Rönnelid J., Miyazono K., Heldin C. H. Enhanced expression of transforming growth factor-beta s and transforming growth factor-beta type II receptor in the synovial tissues of patients with rheumatoid arthritis. Lab Invest. 1994 May;70(5):620–630. [PubMed] [Google Scholar]
- Tetlow L. C., Woolley D. E. Distribution, activation and tryptase/chymase phenotype of mast cells in the rheumatoid lesion. Ann Rheum Dis. 1995 Jul;54(7):549–555. doi: 10.1136/ard.54.7.549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tetlow L. C., Woolley D. E. Mast cells, cytokines, and metalloproteinases at the rheumatoid lesion: dual immunolocalisation studies. Ann Rheum Dis. 1995 Nov;54(11):896–903. doi: 10.1136/ard.54.11.896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Troughton P. R., Platt R., Bird H., el-Manzalawi E., Bassiouni M., Wright V. Synovial fluid interleukin-8 and neutrophil function in rheumatoid arthritis and seronegative polyarthritis. Br J Rheumatol. 1996 Dec;35(12):1244–1251. doi: 10.1093/rheumatology/35.12.1244. [DOI] [PubMed] [Google Scholar]
- Ulfgren A. K., Gröndal L., Lindblad S., Khademi M., Johnell O., Klareskog L., Andersson U. Interindividual and intra-articular variation of proinflammatory cytokines in patients with rheumatoid arthritis: potential implications for treatment. Ann Rheum Dis. 2000 Jun;59(6):439–447. doi: 10.1136/ard.59.6.439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulfgren A. K., Lindblad S., Klareskog L., Andersson J., Andersson U. Detection of cytokine producing cells in the synovial membrane from patients with rheumatoid arthritis. Ann Rheum Dis. 1995 Aug;54(8):654–661. doi: 10.1136/ard.54.8.654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wasserman S. I. The mast cell and synovial inflammation. Or, what's a nice cell like you doing in a joint like this? Arthritis Rheum. 1984 Aug;27(8):841–844. doi: 10.1002/art.1780270801. [DOI] [PubMed] [Google Scholar]
- Wynne-Roberts C. R., Anderson C. H., Turano A. M., Baron M. Light- and electron-microscopic findings of juvenile rheumatoid arthritis synovium: comparison with normal juvenile synovium. Semin Arthritis Rheum. 1978 May;7(4):287–302. doi: 10.1016/0049-0172(78)90027-6. [DOI] [PubMed] [Google Scholar]
- al-Mughales J., Blyth T. H., Hunter J. A., Wilkinson P. C. The chemoattractant activity of rheumatoid synovial fluid for human lymphocytes is due to multiple cytokines. Clin Exp Immunol. 1996 Nov;106(2):230–236. doi: 10.1046/j.1365-2249.1996.d01-836.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Vos S., Brach M. A., Asano Y., Ludwig W. D., Bettelheim P., Gruss H. J., Herrmann F. Transforming growth factor-beta 1 interferes with the proliferation-inducing activity of stem cell factor in myelogenous leukemia blasts through functional down-regulation of the c-kit proto-oncogene product. Cancer Res. 1993 Aug 1;53(15):3638–3642. [PubMed] [Google Scholar]