Abstract
OBJECTIVE—To evaluate the influence of inactivation of one allele ("heterozygous knockout" or "heterozygous inactivation") of the type II procollagen gene (Col2a1) on the biomechanical properties and structure of the articular cartilage and subchondral bone in 15 month old mice. METHODS—Indentation stiffness of the humerus head articular cartilage was measured by a microindentation method. Cartilage and subchondral bone were prepared for digital densitometry of proteoglycans (PGs), polarised light microscopy (PLM) of collagen, and osteoarthrosis (OA) grading. RESULTS—Heterozygous inactivation of the Col2a1 gene softened articular cartilage (p=0.002) as measured by indentation stiffness ((mean (SEM) 0.50 (0.07) MPa v 0.94 (0.13) MPa in controls). Fibrillar collagen network exhibited lower birefringence in the intermediate (p=0.04) and deep zones (p=0.01) of cartilage by PLM, indicating either decreased collagen content or a lower degree of fibril parallelism in the knockout mice. The total and zonal thicknesses of articular cartilage were unchanged. Zonal PG contents did not differ significantly. In knockout mice, the prevalence of superficial fibrillation—that is, a sign of OA, was higher than in controls (73% v 21%, p=0.002). The collagen induced birefringence of the superficial zone was not reduced. The subchondral bone volume fraction was lower in knockout mice than in controls, 31% v 43% (p=0.01), and optical retardation values in PLM of bone collagen were slightly but significantly lower (p=0.01). CONCLUSION—Heterozygous inactivation of the Col2a1 gene made articular cartilage softer, altered the collagenous network, reduced subchondral bone volume, and altered its microstructure. Changes in the cartilage collagen network probably contributed to increased susceptibility to OA.
Full Text
The Full Text of this article is available as a PDF (162.5 KB).
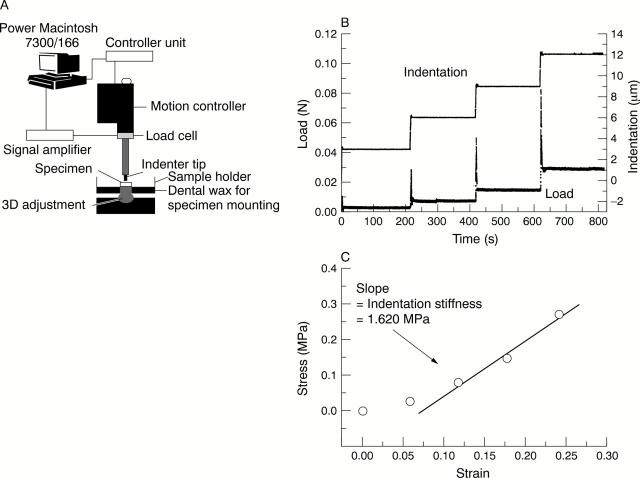
Figure 1 .
(A) Schematic presentation for the indentation measurement setup. (B) A typical series of four consecutive indentation stress relaxation steps (3 µm for each step with ramping speed of 1 µm/s) causing a total strain of 24% in mouse humerus articular cartilage (full thickness about 50 µm). After 200 s of relaxation the next step was taken. (C) Equilibrium stress-strain curve and determination of indentation stiffness from the stress-strain slope.
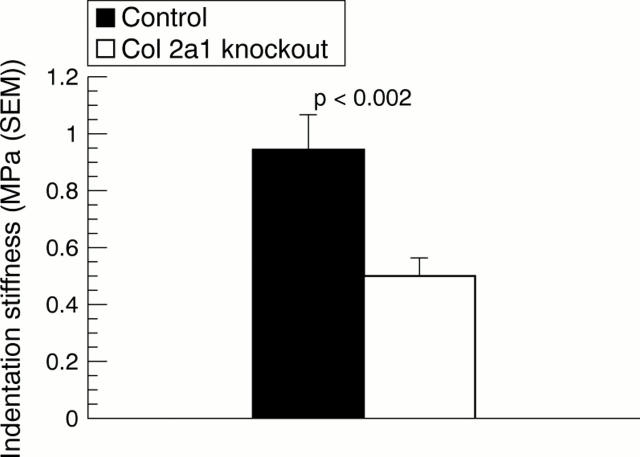
Figure 2 .
Indentation stiffness of articular cartilage (mean (SEM)) in the head of the humerus of the herterozygous Col2a1 knockout and control mice.
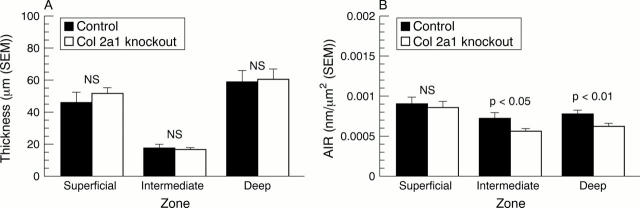
Figure 3 .
(A) Thickness (mean (SEM)) of superficial, intermediate, and deep zones in control and knockout mice. Absolute cartilage thickness is the sum of these three zones. Mann-Whitney U test. (B) Collagen birefringence expressed as area integrated retardation (AIR, mean (SEM)) of semicircularly polarised light in the superficial, intermediate, and deep zones of articular cartilage of heterozygous Col2a1 knockout and control mice. The three zones contain collagen fibrils oriented tangentially, obliquely, and perpendicularly to the surface. Mann-Whitney U test.
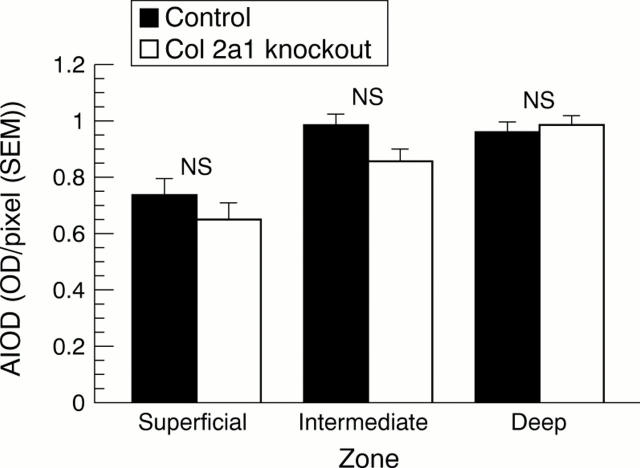
Figure 4 .
Safranin O absorbance expressed as area integrated optical density (AIOD) in superficial, intermediate, and deep zones of articular cartilage. Zones are the same as those in figs 3A and B. Mann-Whitney U test.
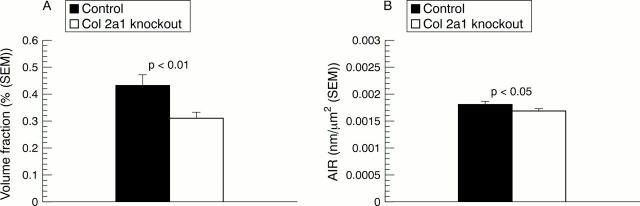
Figure 5 .
(A) Volume fraction of subchondral bone (mean (SEM)) in the proximal humerus. Mann-Whitney U test. (B) Collagen birefringence in subchondral bone expressed as area integrated retardation (AIR, mean (SEM)) of semicircularly polarised light. Mann-Whitney U test.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arokoski J. P., Hyttinen M. M., Helminen H. J., Jurvelin J. S. Biomechanical and structural characteristics of canine femoral and tibial cartilage. J Biomed Mater Res. 1999;48(2):99–107. doi: 10.1002/(sici)1097-4636(1999)48:2<99::aid-jbm1>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- Arokoski J. P., Hyttinen M. M., Lapveteläinen T., Takács P., Kosztáczky B., Módis L., Kovanen V., Helminen H. Decreased birefringence of the superficial zone collagen network in the canine knee (stifle) articular cartilage after long distance running training, detected by quantitative polarised light microscopy. Ann Rheum Dis. 1996 Apr;55(4):253–264. doi: 10.1136/ard.55.4.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aspden R. M., Hukins D. W. Collagen organization in articular cartilage, determined by X-ray diffraction, and its relationship to tissue function. Proc R Soc Lond B Biol Sci. 1981 Jul 14;212(1188):299–304. doi: 10.1098/rspb.1981.0040. [DOI] [PubMed] [Google Scholar]
- Aszódi A., Chan D., Hunziker E., Bateman J. F., Fässler R. Collagen II is essential for the removal of the notochord and the formation of intervertebral discs. J Cell Biol. 1998 Nov 30;143(5):1399–1412. doi: 10.1083/jcb.143.5.1399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bader D. L., Kempson G. E. The short-term compressive properties of adult human articular cartilage. Biomed Mater Eng. 1994;4(3):245–256. [PubMed] [Google Scholar]
- Broom N. D., Silyn-Roberts H. Collagen-collagen versus collagen-proteoglycan interactions in the determination of cartilage strength. Arthritis Rheum. 1990 Oct;33(10):1512–1517. doi: 10.1002/art.1780331008. [DOI] [PubMed] [Google Scholar]
- Broom N. D., Silyn-Roberts H. The three-dimensional 'knit' of collagen fibrils in articular cartilage. Connect Tissue Res. 1989;23(4):261–277. doi: 10.3109/03008208909005626. [DOI] [PubMed] [Google Scholar]
- Busler D. E., Li S. W. Rapid screening of transgenic type II and type XI collagen knock-out mice with three-primer PCR. Biotechniques. 1996 Dec;21(6):1002–1004. doi: 10.2144/96216bm07. [DOI] [PubMed] [Google Scholar]
- Choi K., Kuhn J. L., Ciarelli M. J., Goldstein S. A. The elastic moduli of human subchondral, trabecular, and cortical bone tissue and the size-dependency of cortical bone modulus. J Biomech. 1990;23(11):1103–1113. doi: 10.1016/0021-9290(90)90003-l. [DOI] [PubMed] [Google Scholar]
- Deák F., Piecha D., Bachrati C., Paulsson M., Kiss I. Primary structure and expression of matrilin-2, the closest relative of cartilage matrix protein within the von Willebrand factor type A-like module superfamily. J Biol Chem. 1997 Apr 4;272(14):9268–9274. doi: 10.1074/jbc.272.14.9268. [DOI] [PubMed] [Google Scholar]
- Eyre D. R., Wu J. J. Collagen structure and cartilage matrix integrity. J Rheumatol Suppl. 1995 Feb;43:82–85. [PubMed] [Google Scholar]
- Freeman M. A. Is collagen fatigue failure a cause of osteoarthrosis and prosthetic component migration? A hypothesis. J Orthop Res. 1999 Jan;17(1):3–8. doi: 10.1002/jor.1100170103. [DOI] [PubMed] [Google Scholar]
- Grynpas M. D., Alpert B., Katz I., Lieberman I., Pritzker K. P. Subchondral bone in osteoarthritis. Calcif Tissue Int. 1991 Jul;49(1):20–26. doi: 10.1007/BF02555898. [DOI] [PubMed] [Google Scholar]
- Hardingham T. E., Fosang A. J. Proteoglycans: many forms and many functions. FASEB J. 1992 Feb 1;6(3):861–870. [PubMed] [Google Scholar]
- Hayes W. C., Keer L. M., Herrmann G., Mockros L. F. A mathematical analysis for indentation tests of articular cartilage. J Biomech. 1972 Sep;5(5):541–551. doi: 10.1016/0021-9290(72)90010-3. [DOI] [PubMed] [Google Scholar]
- Helminen H. J., Kiraly K., Pelttari A., Tammi M. I., Vandenberg P., Pereira R., Dhulipala R., Khillan J. S., Ala-Kokko L., Hume E. L. An inbred line of transgenic mice expressing an internally deleted gene for type II procollagen (COL2A1). Young mice have a variable phenotype of a chondrodysplasia and older mice have osteoarthritic changes in joints. J Clin Invest. 1993 Aug;92(2):582–595. doi: 10.1172/JCI116625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoch D. H., Grodzinsky A. J., Koob T. J., Albert M. L., Eyre D. R. Early changes in material properties of rabbit articular cartilage after meniscectomy. J Orthop Res. 1983;1(1):4–12. doi: 10.1002/jor.1100010102. [DOI] [PubMed] [Google Scholar]
- Hollander A. P., Pidoux I., Reiner A., Rorabeck C., Bourne R., Poole A. R. Damage to type II collagen in aging and osteoarthritis starts at the articular surface, originates around chondrocytes, and extends into the cartilage with progressive degeneration. J Clin Invest. 1995 Dec;96(6):2859–2869. doi: 10.1172/JCI118357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang W. S., Li B., Jin L. H., Ngo K., Schachar N. S., Hughes G. N. Collagen fibril structure of normal, aging, and osteoarthritic cartilage. J Pathol. 1992 Aug;167(4):425–433. doi: 10.1002/path.1711670413. [DOI] [PubMed] [Google Scholar]
- Jeffery A. K., Blunn G. W., Archer C. W., Bentley G. Three-dimensional collagen architecture in bovine articular cartilage. J Bone Joint Surg Br. 1991 Sep;73(5):795–801. doi: 10.1302/0301-620X.73B5.1894669. [DOI] [PubMed] [Google Scholar]
- Jurvelin J. S., Buschmann M. D., Hunziker E. B. Optical and mechanical determination of Poisson's ratio of adult bovine humeral articular cartilage. J Biomech. 1997 Mar;30(3):235–241. doi: 10.1016/s0021-9290(96)00133-9. [DOI] [PubMed] [Google Scholar]
- Jurvelin J. S., Räsänen T., Kolmonen P., Lyyra T. Comparison of optical, needle probe and ultrasonic techniques for the measurement of articular cartilage thickness. J Biomech. 1995 Feb;28(2):231–235. doi: 10.1016/0021-9290(94)00060-h. [DOI] [PubMed] [Google Scholar]
- Király K., Hyttinen M. M., Lapveteläinen T., Elo M., Kiviranta I., Dobai J., Módis L., Helminen H. J., Arokoski J. P. Specimen preparation and quantification of collagen birefringence in unstained sections of articular cartilage using image analysis and polarizing light microscopy. Histochem J. 1997 Apr;29(4):317–327. doi: 10.1023/a:1020802631968. [DOI] [PubMed] [Google Scholar]
- Kiviranta I., Jurvelin J., Tammi M., Sämänen A. M., Helminen H. J. Microspectrophotometric quantitation of glycosaminoglycans in articular cartilage sections stained with Safranin O. Histochemistry. 1985;82(3):249–255. doi: 10.1007/BF00501401. [DOI] [PubMed] [Google Scholar]
- Kuivaniemi H., Tromp G., Prockop D. J. Mutations in fibrillar collagens (types I, II, III, and XI), fibril-associated collagen (type IX), and network-forming collagen (type X) cause a spectrum of diseases of bone, cartilage, and blood vessels. Hum Mutat. 1997;9(4):300–315. doi: 10.1002/(SICI)1098-1004(1997)9:4<300::AID-HUMU2>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- Käb M. J., Gwynn I. A., Nötzli H. P. Collagen fibre arrangement in the tibial plateau articular cartilage of man and other mammalian species. J Anat. 1998 Jul;193(Pt 1):23–34. doi: 10.1046/j.1469-7580.1998.19310023.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Käb M. J., Ito K., Clark J. M., Nötzli H. P. Deformation of articular cartilage collagen structure under static and cyclic loading. J Orthop Res. 1998 Nov;16(6):743–751. doi: 10.1002/jor.1100160617. [DOI] [PubMed] [Google Scholar]
- Lapveteläinen T., Nevalainen T., Parkkinen J. J., Arokoski J., Kiraly K., Hyttinen M., Halonen P., Helminen H. J. Lifelong moderate running training increases the incidence and severity of osteoarthritis in the knee joint of C57BL mice. Anat Rec. 1995 Jun;242(2):159–165. doi: 10.1002/ar.1092420204. [DOI] [PubMed] [Google Scholar]
- Li S. W., Prockop D. J., Helminen H., Fässler R., Lapveteläinen T., Kiraly K., Peltarri A., Arokoski J., Lui H., Arita M. Transgenic mice with targeted inactivation of the Col2 alpha 1 gene for collagen II develop a skeleton with membranous and periosteal bone but no endochondral bone. Genes Dev. 1995 Nov 15;9(22):2821–2830. doi: 10.1101/gad.9.22.2821. [DOI] [PubMed] [Google Scholar]
- Mak A. F., Lai W. M., Mow V. C. Biphasic indentation of articular cartilage--I. Theoretical analysis. J Biomech. 1987;20(7):703–714. doi: 10.1016/0021-9290(87)90036-4. [DOI] [PubMed] [Google Scholar]
- Mendler M., Eich-Bender S. G., Vaughan L., Winterhalter K. H., Bruckner P. Cartilage contains mixed fibrils of collagen types II, IX, and XI. J Cell Biol. 1989 Jan;108(1):191–197. doi: 10.1083/jcb.108.1.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metsäranta M., Garofalo S., Decker G., Rintala M., de Crombrugghe B., Vuorio E. Chondrodysplasia in transgenic mice harboring a 15-amino acid deletion in the triple helical domain of pro alpha 1(II) collagen chain. J Cell Biol. 1992 Jul;118(1):203–212. doi: 10.1083/jcb.118.1.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison E. H., Ferguson M. W., Bayliss M. T., Archer C. W. The development of articular cartilage: I. The spatial and temporal patterns of collagen types. J Anat. 1996 Aug;189(Pt 1):9–22. [PMC free article] [PubMed] [Google Scholar]
- Mow V. C., Ratcliffe A., Poole A. R. Cartilage and diarthrodial joints as paradigms for hierarchical materials and structures. Biomaterials. 1992;13(2):67–97. doi: 10.1016/0142-9612(92)90001-5. [DOI] [PubMed] [Google Scholar]
- Panula H. E., Hyttinen M. M., Arokoski J. P., Långsjö T. K., Pelttari A., Kiviranta I., Helminen H. J. Articular cartilage superficial zone collagen birefringence reduced and cartilage thickness increased before surface fibrillation in experimental osteoarthritis. Ann Rheum Dis. 1998 Apr;57(4):237–245. doi: 10.1136/ard.57.4.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prockop D. J., Kivirikko K. I. Collagens: molecular biology, diseases, and potentials for therapy. Annu Rev Biochem. 1995;64:403–434. doi: 10.1146/annurev.bi.64.070195.002155. [DOI] [PubMed] [Google Scholar]
- Speer D. P., Dahners L. The collagenous architecture of articular cartilage. Correlation of scanning electron microscopy and polarized light microscopy observations. Clin Orthop Relat Res. 1979 Mar-Apr;(139):267–275. [PubMed] [Google Scholar]
- Vikkula M., Metsäranta M., Ala-Kokko L. Type II collagen mutations in rare and common cartilage diseases. Ann Med. 1994 Apr;26(2):107–114. doi: 10.3109/07853899409147337. [DOI] [PubMed] [Google Scholar]