Abstract
OBJECTIVE—To examine the hypothesis that fetal microchimerism plays a part in the pathogenic process of Sjögren's syndrome (SS). METHODS—Genomic DNA samples were extracted from peripheral blood whole nucleated cells and the CD34+ cell enriched fraction of patients with SS and healthy women who had male offspring as well as nulliparous women. A Y chromosome-specific sequence was detected as a marker for fetal cells by a nested polymerase chain reaction (PCR) and by DNA hybridisation combined with PCR using specific primers and probes. All procedures were performed with great care to avoid the contamination of male DNA. RESULTS—A nested PCR and DNA hybridisation combined with PCR was established that can detect a single male cell out of 1.67×105 female cells. It was not possible to increase the sensitivity further because the amount of template DNA held in the PCR was limited. When these methods were used, no fetal cells were detected in any samples from patients with SS, though they were detected in whole nucleated cells from two healthy women who had delivered sons previously. CONCLUSIONS—The findings indicate that circulating fetal cells in patients with SS are uncommon (<1 in 1.67×105), if they exist. With the conventional PCR based methods that were used, it is difficult to evaluate the quantitative difference in circulating fetal cells between patients with SS and healthy women.
Full Text
The Full Text of this article is available as a PDF (176.6 KB).
Figure 1 .

Amplification of the TSPY gene using male DNA as a template in the presence of serial amounts of background female DNA. Serial amounts of female genomic DNA (100 ng—10 µg) were mixed with 10 ng male genomic DNA and used as templates for the first polymerase chain reaction (PCR). The PCR products were fractionated on a 1.5% agarose gel and stained with ethidium bromide. Lane 1: 100 ng, lane 2: 300 ng, lane 3: 600 ng, lane 4: 1 µg, lane 5: 2 µg, lane 6: 5 µg, and lane 7: 10 µg of background female DNA. Lane 8: molecular weight markers (100 bp ladder).
Figure 2 .
Detection of the TSPY gene by nested polymerase chain reaction (PCR) using serial amounts of male DNA as templates. Serial amounts of male DNA (10−3-10−10 µg) in 1 µg of female DNA were amplified by the nested PCR. The PCR products were fractionated on 1.5% agarose gel and stained with ethidium bromide. Lane 1: 10−3 µg, lane 2: 10−4 µg, lane 3: 10−5 µg, lane 4: 10−6 µg, lane 5: 10−7 µg, lane 6: 10−8 µg, lane 7: 10−9 µg, and lane 8: 10−10 µg of male DNA. Lane 9: no template for the first PCR and lane 10: no template for the second PCR. Lane 11: molecular weight markers (100 bp ladder).
Figure 3 .
Detection of male cells in peripheral blood whole nucleated cells from women who had delivered sons within the past 24 hours by a nested polymerase chain reaction (PCR). Genomic DNA from peripheral blood whole nucleated cells obtained from three women one day after delivery (lanes 1-3: 1 µg), and control male DNA (lane 4: 10−5 µg and lane 5: 10−2 µg) were applied for the nested PCR. The PCR products were fractionated on 1.5% agarose gel and stained with ethidium bromide. Lane 6: no template for the first PCR and lane 7: no template for the second PCR. Lane 8: molecular weight markers (100 bp ladder).
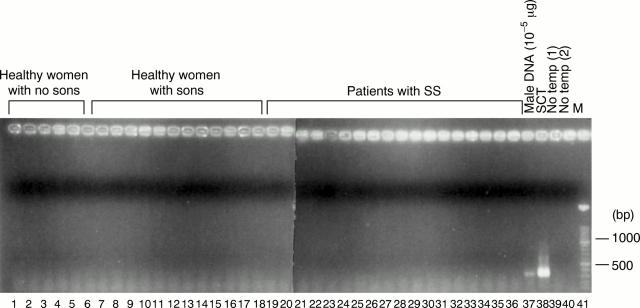
Figure 4 .
Detection of male cells in peripheral blood whole nucleated cells from healthy women with no sons, healthy women with sons, and patients with SS with sons by a nested polymerase chain reaction (PCR). Genomic DNA (1 µg) from these subjects was amplified by a nested PCR, and the PCR products were fractionated on 1.5% agarose gel and stained with ethidium bromide. Lanes 1-6: healthy women with no sons, lanes 7-17: healthy women with sons, and lanes 18-36: patients with SS with sons. Lane 37: male DNA (10−5 µg), lane 38: DNA from a female stem cell transplantation (SCT) recipient from a male donor (1 µg), lane 39: no template for the first PCR, and lane 40: no template for the second PCR. Lane 41: molecular weight markers (100 bp ladder).
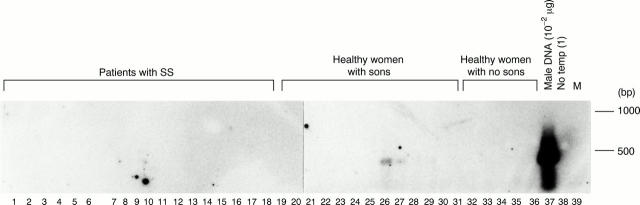
Figure 5 .
Detection of male cells in peripheral blood whole nucleated cells from patients with Sjögren's syndrome with sons, healthy women with sons, and healthy women with no sons by DNA hybridisation combined with a polymerase chain reaction (PCR). The PCR products of the first PCR were fractionated by electrophoresis and hybridised with probes specific for the TSPY gene. Reactivities were visualised with a chemiluminescence detection system. Lanes 1-18: patients with SS with sons, lanes 19-31: healthy women with sons, and lanes 32-36: healthy women with no sons. Lane 37: male DNA (10−2 µg) in the first PCR and lane 38: no template for the first PCR. Lane 39: molecular weight markers (100 bp ladder).
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ahmed S. A., Aufdemorte T. B., Chen J. R., Montoya A. I., Olive D., Talal N. Estrogen induces the development of autoantibodies and promotes salivary gland lymphoid infiltrates in normal mice. J Autoimmun. 1989 Aug;2(4):543–552. doi: 10.1016/0896-8411(89)90187-x. [DOI] [PubMed] [Google Scholar]
- Ariga H., Edwards J., Sullivan D. A. Androgen control of autoimmune expression in lacrimal glands of MRL/Mp-lpr/lpr mice. Clin Immunol Immunopathol. 1989 Dec;53(3):499–508. doi: 10.1016/0090-1229(89)90011-1. [DOI] [PubMed] [Google Scholar]
- Artlett C. M., Smith J. B., Jimenez S. A. Identification of fetal DNA and cells in skin lesions from women with systemic sclerosis. N Engl J Med. 1998 Apr 23;338(17):1186–1191. doi: 10.1056/NEJM199804233381704. [DOI] [PubMed] [Google Scholar]
- Atkinson K. Chronic graft-versus-host disease. Bone Marrow Transplant. 1990 Feb;5(2):69–82. [PubMed] [Google Scholar]
- Beeson P. B. Age and sex associations of 40 autoimmune diseases. Am J Med. 1994 May;96(5):457–462. doi: 10.1016/0002-9343(94)90173-2. [DOI] [PubMed] [Google Scholar]
- Bianchi D. W., Zickwolf G. K., Weil G. J., Sylvester S., DeMaria M. A. Male fetal progenitor cells persist in maternal blood for as long as 27 years postpartum. Proc Natl Acad Sci U S A. 1996 Jan 23;93(2):705–708. doi: 10.1073/pnas.93.2.705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlsten H., Nilsson N., Jonsson R., Bäckman K., Holmdahl R., Tarkowski A. Estrogen accelerates immune complex glomerulonephritis but ameliorates T cell-mediated vasculitis and sialadenitis in autoimmune MRL lpr/lpr mice. Cell Immunol. 1992 Oct 1;144(1):190–202. doi: 10.1016/0008-8749(92)90236-i. [DOI] [PubMed] [Google Scholar]
- Evans P. C., Lambert N., Maloney S., Furst D. E., Moore J. M., Nelson J. L. Long-term fetal microchimerism in peripheral blood mononuclear cell subsets in healthy women and women with scleroderma. Blood. 1999 Mar 15;93(6):2033–2037. [PubMed] [Google Scholar]
- Fox R. I., Pearson G., Vaughan J. H. Detection of Epstein-Barr virus-associated antigens and DNA in salivary gland biopsies from patients with Sjogren's syndrome. J Immunol. 1986 Nov 15;137(10):3162–3168. [PubMed] [Google Scholar]
- Fox R. I., Robinson C. A., Curd J. G., Kozin F., Howell F. V. Sjögren's syndrome. Proposed criteria for classification. Arthritis Rheum. 1986 May;29(5):577–585. doi: 10.1002/art.1780290501. [DOI] [PubMed] [Google Scholar]
- Fox R. I., Saito I. Sjögren's syndrome: immunologic and neuroendocrine mechanisms. Adv Exp Med Biol. 1994;350:609–621. doi: 10.1007/978-1-4615-2417-5_103. [DOI] [PubMed] [Google Scholar]
- Hamada H., Arinami T., Hamaguchi H., Kubo T. Fetal nucleated cells in maternal peripheral blood after delivery. Am J Obstet Gynecol. 1994 Apr;170(4):1188–1193. doi: 10.1016/s0002-9378(94)70120-2. [DOI] [PubMed] [Google Scholar]
- Harley J. B., Alexander E. L., Bias W. B., Fox O. F., Provost T. T., Reichlin M., Yamagata H., Arnett F. C. Anti-Ro (SS-A) and anti-La (SS-B) in patients with Sjögren's syndrome. Arthritis Rheum. 1986 Feb;29(2):196–206. doi: 10.1002/art.1780290207. [DOI] [PubMed] [Google Scholar]
- Homo-Delarche F., Fitzpatrick F., Christeff N., Nunez E. A., Bach J. F., Dardenne M. Sex steroids, glucocorticoids, stress and autoimmunity. J Steroid Biochem Mol Biol. 1991;40(4-6):619–637. doi: 10.1016/0960-0760(91)90285-d. [DOI] [PubMed] [Google Scholar]
- Ishimaru N., Saegusa K., Yanagi K., Haneji N., Saito I., Hayashi Y. Estrogen deficiency accelerates autoimmune exocrinopathy in murine Sjögren's syndrome through fas-mediated apoptosis. Am J Pathol. 1999 Jul;155(1):173–181. doi: 10.1016/S0002-9440(10)65111-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeffreys A. J., Wilson V., Neumann R., Keyte J. Amplification of human minisatellites by the polymerase chain reaction: towards DNA fingerprinting of single cells. Nucleic Acids Res. 1988 Dec 9;16(23):10953–10971. doi: 10.1093/nar/16.23.10953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawley T. J., Peck G. L., Moutsopoulos H. M., Gratwohl A. A., Deisseroth A. B. Scleroderma, Sjögren-like syndrome, and chronic graft-versus-host disease. Ann Intern Med. 1977 Dec;87(6):707–709. doi: 10.7326/0003-4819-87-6-707. [DOI] [PubMed] [Google Scholar]
- McFarlane I. G., McFarlane B. M., Haines A. J., Eddleston A. L., Williams R. Relationship between primary biliary cirrhosis and chronic graft versus host disease: investigation of histocompatibility (HLA) antigenic determinants in biliary tract antigens. Clin Sci (Lond) 1983 Jan;64(1):113–116. doi: 10.1042/cs0640113. [DOI] [PubMed] [Google Scholar]
- Murata H., Nakauchi H., Sumida T. Microchimerism in Japanese women patients with systemic sclerosis. Lancet. 1999 Jul 17;354(9174):220–220. doi: 10.1016/S0140-6736(99)00164-6. [DOI] [PubMed] [Google Scholar]
- Nelson J. L., Furst D. E., Maloney S., Gooley T., Evans P. C., Smith A., Bean M. A., Ober C., Bianchi D. W. Microchimerism and HLA-compatible relationships of pregnancy in scleroderma. Lancet. 1998 Feb 21;351(9102):559–562. doi: 10.1016/S0140-6736(97)08357-8. [DOI] [PubMed] [Google Scholar]
- Ogawa Y., Okamoto S., Wakui M., Watanabe R., Yamada M., Yoshino M., Ono M., Yang H. Y., Mashima Y., Oguchi Y. Dry eye after haematopoietic stem cell transplantation. Br J Ophthalmol. 1999 Oct;83(10):1125–1130. doi: 10.1136/bjo.83.10.1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubbia-Brandt L., Philippeaux M. M., Chavez S., Mentha G., Borisch B., Hadengue A. FISH for Y chromosome in women with primary biliary cirrhosis: lack of evidence for leukocyte microchimerism. Hepatology. 1999 Sep;30(3):821–822. doi: 10.1002/hep.510300322. [DOI] [PubMed] [Google Scholar]
- Sahota A., Yang M., McDaniel H. B., Sidner R. A., Book B., Barr R., Brahmi Z., Jindal R. M. Evaluation of seven PCR-based assays for the analysis of microchimerism. Clin Biochem. 1998 Nov;31(8):641–645. doi: 10.1016/s0009-9120(98)00059-9. [DOI] [PubMed] [Google Scholar]
- Schuurs A. H., Verheul H. A. Effects of gender and sex steroids on the immune response. J Steroid Biochem. 1990 Feb;35(2):157–172. doi: 10.1016/0022-4731(90)90270-3. [DOI] [PubMed] [Google Scholar]
- Tyndall A., Gratwohl A. Microchimerism: friend or foe? Nat Med. 1998 Apr;4(4):386–388. doi: 10.1038/nm0498-386. [DOI] [PubMed] [Google Scholar]
- Vitali C., Bombardieri S., Moutsopoulos H. M., Balestrieri G., Bencivelli W., Bernstein R. M., Bjerrum K. B., Braga S., Coll J., de Vita S. Preliminary criteria for the classification of Sjögren's syndrome. Results of a prospective concerted action supported by the European Community. Arthritis Rheum. 1993 Mar;36(3):340–347. doi: 10.1002/art.1780360309. [DOI] [PubMed] [Google Scholar]
- Vogel T., Schmidtke J. Structure and function of TSPY, the Y-chromosome gene coding for the "testis-specific protein". Cytogenet Cell Genet. 1998;80(1-4):209–213. doi: 10.1159/000014982. [DOI] [PubMed] [Google Scholar]
- Vázquez-Abad D., Rothfield N. F. Sensitivity and specificity of anti-Jo-1 antibodies in autoimmune diseases with myositis. Arthritis Rheum. 1996 Feb;39(2):292–296. doi: 10.1002/art.1780390218. [DOI] [PubMed] [Google Scholar]