Abstract
OBJECTIVE—To investigate the effect of insulin-like growth factor 1 (IGF1) on the release of collagen, and the production and expression of matrix metalloproteinases (MMPs) induced by the proinflammatory cytokine interleukin 1α (IL1α) in combination with oncostatin M (OSM) from bovine nasal cartilage and primary human articular chondrocytes. METHODS—Human articular chondrocytes and bovine nasal cartilage were cultured with and without IGF1 in the presence of IL1α or IL1α + OSM. The release of collagen was measured by an assay for hydroxyproline. Collagenase activity was determined with the diffuse fibril assay using 3H acetylated collagen. The expression of MMP-1, MMP-3, MMP-8, MMP-13, and tissue inhibitor of metalloproteinase 1 (TIMP-1) mRNA was analysed by northern blot. RESULTS—IGF1 can partially inhibit the release of collagen induced by IL1α or IL1α + OSM from bovine nasal cartilage. This was accompanied by a reduced secretion and activation of collagenase by bovine nasal cartilage. IGF1 can also down regulate IL1α or IL1α + OSM induced MMP-1, MMP-3, MMP-8, and MMP-13 mRNA expression in human articular chondrocytes and bovine chondrocytes. It had no significant effect on the production and expression of TIMP-1 mRNA in chondrocytes. CONCLUSION—This study shows for the first time that IGF1 can partially block the release of collagen from cartilage and suggests that down regulation of collagenases by IGF1 may be an important mechanism in preventing cartilage resorption initiated by proinflammatory cytokines.
Full Text
The Full Text of this article is available as a PDF (204.8 KB).
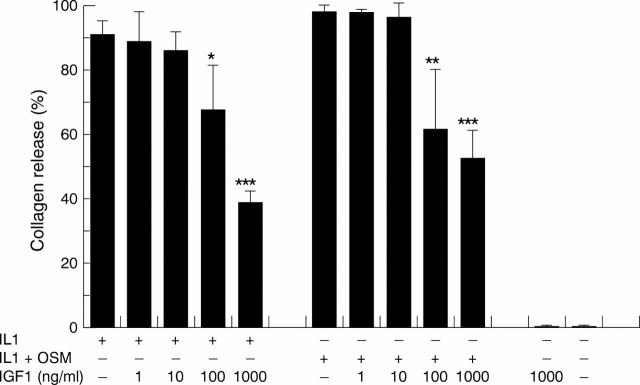
Figure 1 .
The effects of insulin-like growth factor 1 (IGF1) on the release of collagen fragments induced by interleukin 1α (IL1α) and IL1α + oncostatin M (OSM). Bovine nasal cartilage was cultured in serum-free medium in the presence of either medium alone, or medium containing IL1α (1 ng/ml), IL1α (0.2 ng/ml) + OSM (2 ng/ml) with IGF1 (1-1000 ng/ml) for 14 days. Media were collected at days 7 and 14. The levels of collagen fragments released into the media on days 7 and 14 were determined by the measurement of hydroxyproline as described in "Material and methods". Results shown are for the cumulative collagen release for 14 days of culture and expressed as a percentage of the total (mean (SD)). All assays were done in quadruplicate. The experiments were performed in duplicate. Significance was analysed with respect to the cytokine compared with the cytokine + IGF1, or IGF1 compared with control. *p<0.05, **p<0.01, ***p<0.001 using t test.
Figure 2 .
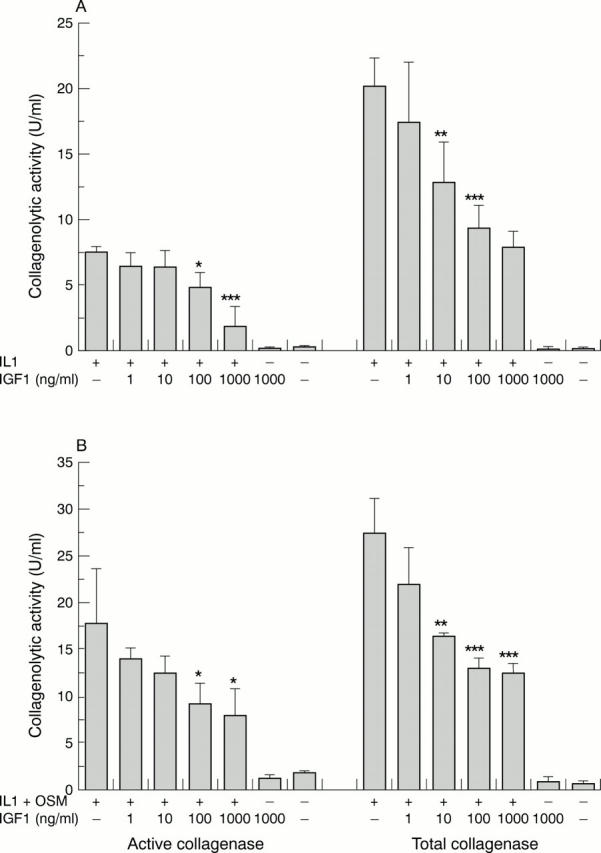
Effects of insulin-like growth factor 1 (IGF1) on the levels of collagenolytic activity induced by interleukin 1α (IL1α) or IL1α + oncostatin M (OSM). Bovine nasal cartilage was treated as described for fig 1. The levels of procollagenase and active collagenase activity in media removed from cultured cartilage at day 14 after stimulation with (A) IL1α and (B) IL1α + OSM, with or without IGF1, were measured as described in "Material and methods".
Figure 3 .
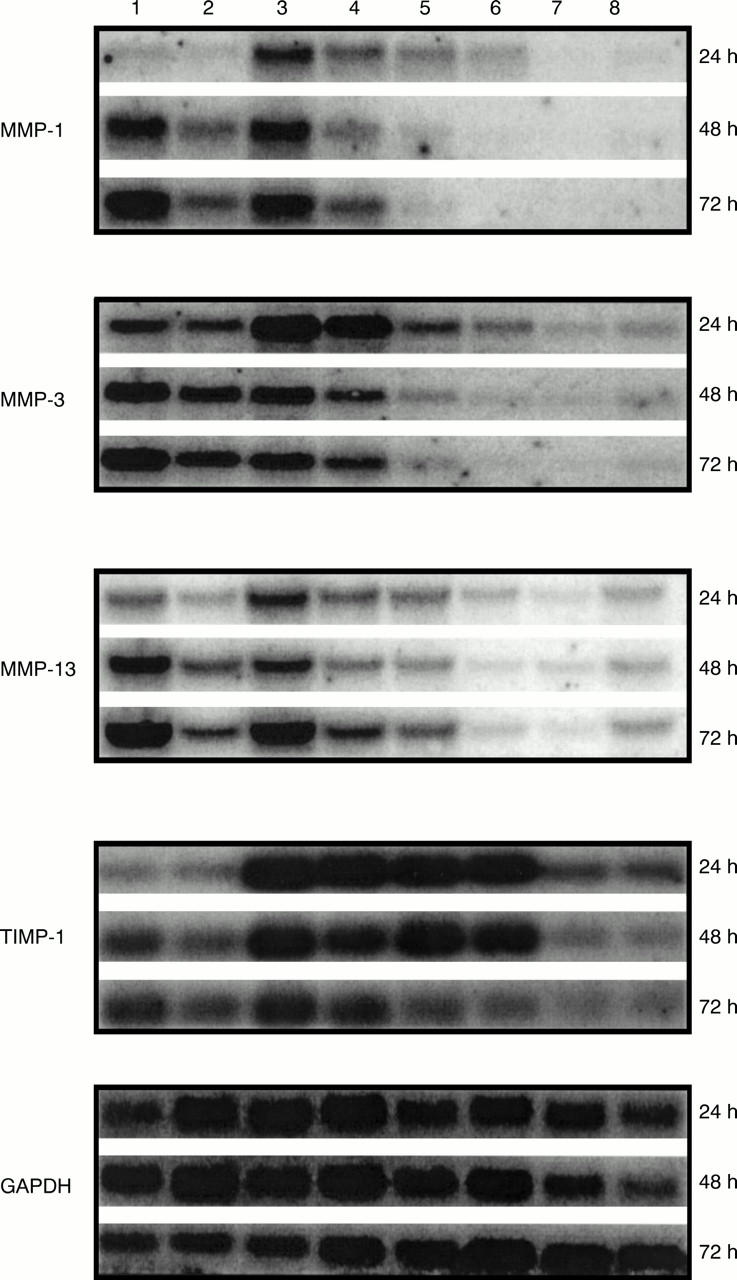
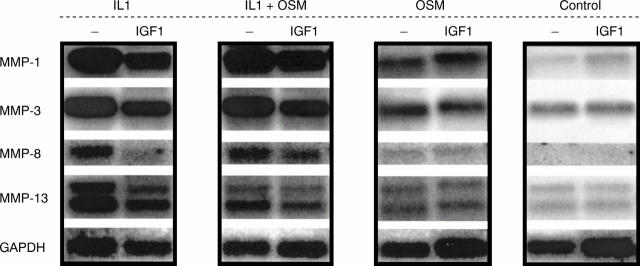
Effects of insulin-like growth factor 1 (IGF1) on the expression of metalloproteinase (MMP) and tissue inhibitor of metalloproteinase (TIMP) mRNA in bovine chondrocytes. Bovine nasal chondrocytes were incubated with interleukin 1α (IL1α; 1 ng/ml), oncostatin M (OSM; 10 ng/ml) and IL1α (0.2 ng/ml) + OSM (2 ng/ml) with IGF1 (200 ng/ml) in serum free medium for the times indicated. The expression of MMP-1, MMP-3, MMP-8, MMP-13, and TIMP-1 mRNA was analysed by northern blot. Lane 1 = IL1α ; lane 2 = IL1α + IGF1; lane 3 = IL1α + OSM; lane 4 = IL1α + OSM + IGF1; lane 5 = OSM; lane 6 = OSM + IGF1; lane 7 = IGF1; lane 8 = control.
Figure 4 .
Effects of insulin-like growth factor 1 (IGF1) on the expression of metalloproteinase (MMP) mRNA in human articular chondrocytes. Human articular chondrocytes were incubated with interleukin 1α (IL1α; 1 ng/ml), oncostatin M (OSM; 10 ng/ml), and IL1α (1 ng/ml) + OSM (10 ng/ml) with IGF1 (200 ng/ml) in serum-free medium for 24 hours. The expression of MMP-1, MMP-3, MMP-8, and MMP-13 mRNA was analysed by northern blot.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andrews H. J., Bunning R. A., Plumpton T. A., Clark I. M., Russell R. G., Cawston T. E. Inhibition of interleukin-1-induced collagenase production in human articular chondrocytes in vitro by recombinant human interferon-gamma. Arthritis Rheum. 1990 Nov;33(11):1733–1738. doi: 10.1002/art.1780331119. [DOI] [PubMed] [Google Scholar]
- Andrews H. J., Edwards T. A., Cawston T. E., Hazleman B. L. Transforming growth factor-beta causes partial inhibition of interleukin 1-stimulated cartilage degradation in vitro. Biochem Biophys Res Commun. 1989 Jul 14;162(1):144–150. doi: 10.1016/0006-291x(89)91974-8. [DOI] [PubMed] [Google Scholar]
- Billinghurst R. C., Dahlberg L., Ionescu M., Reiner A., Bourne R., Rorabeck C., Mitchell P., Hambor J., Diekmann O., Tschesche H. Enhanced cleavage of type II collagen by collagenases in osteoarthritic articular cartilage. J Clin Invest. 1997 Apr 1;99(7):1534–1545. doi: 10.1172/JCI119316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borden P., Solymar D., Sucharczuk A., Lindman B., Cannon P., Heller R. A. Cytokine control of interstitial collagenase and collagenase-3 gene expression in human chondrocytes. J Biol Chem. 1996 Sep 20;271(38):23577–23581. doi: 10.1074/jbc.271.38.23577. [DOI] [PubMed] [Google Scholar]
- Brown T. J., Lioubin M. N., Marquardt H. Purification and characterization of cytostatic lymphokines produced by activated human T lymphocytes. Synergistic antiproliferative activity of transforming growth factor beta 1, interferon-gamma, and oncostatin M for human melanoma cells. J Immunol. 1987 Nov 1;139(9):2977–2983. [PubMed] [Google Scholar]
- Canalis E., Rydziel S., Delany A. M., Varghese S., Jeffrey J. J. Insulin-like growth factors inhibit interstitial collagenase synthesis in bone cell cultures. Endocrinology. 1995 Apr;136(4):1348–1354. doi: 10.1210/endo.136.4.7895645. [DOI] [PubMed] [Google Scholar]
- Cawston T. E., Curry V. A., Summers C. A., Clark I. M., Riley G. P., Life P. F., Spaull J. R., Goldring M. B., Koshy P. J., Rowan A. D. The role of oncostatin M in animal and human connective tissue collagen turnover and its localization within the rheumatoid joint. Arthritis Rheum. 1998 Oct;41(10):1760–1771. doi: 10.1002/1529-0131(199810)41:10<1760::AID-ART8>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- Cawston T. E., Ellis A. J., Bigg H., Curry V., Lean E., Ward D. Interleukin-4 blocks the release of collagen fragments from bovine nasal cartilage treated with cytokines. Biochim Biophys Acta. 1996 Dec 12;1314(3):226–232. doi: 10.1016/s0167-4889(96)00107-3. [DOI] [PubMed] [Google Scholar]
- Cawston T. E., Ellis A. J., Humm G., Lean E., Ward D., Curry V. Interleukin-1 and oncostatin M in combination promote the release of collagen fragments from bovine nasal cartilage in culture. Biochem Biophys Res Commun. 1995 Oct 4;215(1):377–385. doi: 10.1006/bbrc.1995.2476. [DOI] [PubMed] [Google Scholar]
- Cawston T. E. Metalloproteinase inhibitors and the prevention of connective tissue breakdown. Pharmacol Ther. 1996;70(3):163–182. doi: 10.1016/0163-7258(96)00015-0. [DOI] [PubMed] [Google Scholar]
- Cawston T. Matrix metalloproteinases and TIMPs: properties and implications for the rheumatic diseases. Mol Med Today. 1998 Mar;4(3):130–137. doi: 10.1016/s1357-4310(97)01192-1. [DOI] [PubMed] [Google Scholar]
- Chandrasekhar S., Harvey A. K., Stack S. T. Degradative and repair responses of cartilage to cytokines and growth factors occur via distinct pathways. Agents Actions Suppl. 1993;39:121–125. doi: 10.1007/978-3-0348-7442-7_13. [DOI] [PubMed] [Google Scholar]
- Cole A. A., Chubinskaya S., Schumacher B., Huch K., Szabo G., Yao J., Mikecz K., Hasty K. A., Kuettner K. E. Chondrocyte matrix metalloproteinase-8. Human articular chondrocytes express neutrophil collagenase. J Biol Chem. 1996 May 3;271(18):11023–11026. doi: 10.1074/jbc.271.18.11023. [DOI] [PubMed] [Google Scholar]
- Cowell S., Knäuper V., Stewart M. L., D'Ortho M. P., Stanton H., Hembry R. M., López-Otín C., Reynolds J. J., Murphy G. Induction of matrix metalloproteinase activation cascades based on membrane-type 1 matrix metalloproteinase: associated activation of gelatinase A, gelatinase B and collagenase 3. Biochem J. 1998 Apr 15;331(Pt 2):453–458. doi: 10.1042/bj3310453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demarquay D., Dumontier M. F., Tsagris L., Bourguignon J., Nataf V., Corvol M. T. In vitro insulin-like growth factor I interaction with cartilage cells derived from postnatal animals. Horm Res. 1990;33(2-4):111–115. doi: 10.1159/000181493. [DOI] [PubMed] [Google Scholar]
- Doré S., Pelletier J. P., DiBattista J. A., Tardif G., Brazeau P., Martel-Pelletier J. Human osteoarthritic chondrocytes possess an increased number of insulin-like growth factor 1 binding sites but are unresponsive to its stimulation. Possible role of IGF-1-binding proteins. Arthritis Rheum. 1994 Feb;37(2):253–263. doi: 10.1002/art.1780370215. [DOI] [PubMed] [Google Scholar]
- Farndale R. W., Buttle D. J., Barrett A. J. Improved quantitation and discrimination of sulphated glycosaminoglycans by use of dimethylmethylene blue. Biochim Biophys Acta. 1986 Sep 4;883(2):173–177. doi: 10.1016/0304-4165(86)90306-5. [DOI] [PubMed] [Google Scholar]
- Fowlkes J. L., Serra D. M., Nagase H., Thrailkill K. M. MMPs are IGFBP-degrading proteinases: implications for cell proliferation and tissue growth. Ann N Y Acad Sci. 1999 Jun 30;878:696–699. doi: 10.1111/j.1749-6632.1999.tb07765.x. [DOI] [PubMed] [Google Scholar]
- Froesch E. R., Schmid C., Schwander J., Zapf J. Actions of insulin-like growth factors. Annu Rev Physiol. 1985;47:443–467. doi: 10.1146/annurev.ph.47.030185.002303. [DOI] [PubMed] [Google Scholar]
- Gadher S. J., Eyre D. R., Wotton S. F., Schmid T. M., Woolley D. E. Degradation of cartilage collagens type II, IX, X and XI by enzymes derived from human articular chondrocytes. Matrix. 1990 Jul;10(3):154–163. doi: 10.1016/s0934-8832(11)80164-2. [DOI] [PubMed] [Google Scholar]
- Goldring M. B., Birkhead J., Sandell L. J., Kimura T., Krane S. M. Interleukin 1 suppresses expression of cartilage-specific types II and IX collagens and increases types I and III collagens in human chondrocytes. J Clin Invest. 1988 Dec;82(6):2026–2037. doi: 10.1172/JCI113823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Günther M., Haubeck H. D., van de Leur E., Bläser J., Bender S., Gütgemann I., Fischer D. C., Tschesche H., Greiling H., Heinrich P. C. Transforming growth factor beta 1 regulates tissue inhibitor of metalloproteinases-1 expression in differentiated human articular chondrocytes. Arthritis Rheum. 1994 Mar;37(3):395–405. doi: 10.1002/art.1780370314. [DOI] [PubMed] [Google Scholar]
- Hardingham T. E., Fosang A. J. The structure of aggrecan and its turnover in cartilage. J Rheumatol Suppl. 1995 Feb;43:86–90. [PubMed] [Google Scholar]
- Hascall V. C., Handley C. J., McQuillan D. J., Hascall G. K., Robinson H. C., Lowther D. A. The effect of serum on biosynthesis of proteoglycans by bovine articular cartilage in culture. Arch Biochem Biophys. 1983 Jul 1;224(1):206–223. doi: 10.1016/0003-9861(83)90205-9. [DOI] [PubMed] [Google Scholar]
- Hui W., Bell M., Carroll G. Detection of oncostatin M in synovial fluid from patients with rheumatoid arthritis. Ann Rheum Dis. 1997 Mar;56(3):184–187. doi: 10.1136/ard.56.3.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hui W., Bell M., Carroll G. Oncostatin M (OSM) stimulates resorption and inhibits synthesis of proteoglycan in porcine articular cartilage explants. Cytokine. 1996 Jun;8(6):495–500. doi: 10.1006/cyto.1996.0067. [DOI] [PubMed] [Google Scholar]
- Hui W., Rowan A. D., Cawston T. Transforming growth factor beta1 blocks the release of collagen fragments from boving nasal cartilage stimulated by oncostatin M in combination with IL-1alpha. Cytokine. 2000 Jun;12(6):765–769. doi: 10.1006/cyto.1999.0625. [DOI] [PubMed] [Google Scholar]
- Koshy P. J., Rowan A. D., Life P. F., Cawston T. E. 96-Well plate assays for measuring collagenase activity using (3)H-acetylated collagen. Anal Biochem. 1999 Nov 15;275(2):202–207. doi: 10.1006/abio.1999.4310. [DOI] [PubMed] [Google Scholar]
- Luyten F. P., Hascall V. C., Nissley S. P., Morales T. I., Reddi A. H. Insulin-like growth factors maintain steady-state metabolism of proteoglycans in bovine articular cartilage explants. Arch Biochem Biophys. 1988 Dec;267(2):416–425. doi: 10.1016/0003-9861(88)90047-1. [DOI] [PubMed] [Google Scholar]
- McCarthy T. L., Centrella M., Canalis E. Regulatory effects of insulin-like growth factors I and II on bone collagen synthesis in rat calvarial cultures. Endocrinology. 1989 Jan;124(1):301–309. doi: 10.1210/endo-124-1-301. [DOI] [PubMed] [Google Scholar]
- McQuillan D. J., Handley C. J., Campbell M. A., Bolis S., Milway V. E., Herington A. C. Stimulation of proteoglycan biosynthesis by serum and insulin-like growth factor-I in cultured bovine articular cartilage. Biochem J. 1986 Dec 1;240(2):423–430. doi: 10.1042/bj2400423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Middleton J. F., Tyler J. A. Upregulation of insulin-like growth factor I gene expression in the lesions of osteoarthritic human articular cartilage. Ann Rheum Dis. 1992 Apr;51(4):440–447. doi: 10.1136/ard.51.4.440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morales T. I. The role and content of endogenous insulin-like growth factor-binding proteins in bovine articular cartilage. Arch Biochem Biophys. 1997 Jul 15;343(2):164–172. doi: 10.1006/abbi.1997.0166. [DOI] [PubMed] [Google Scholar]
- Morales T. I. Transforming growth factor-beta and insulin-like growth factor-1 restore proteoglycan metabolism of bovine articular cartilage after depletion by retinoic acid. Arch Biochem Biophys. 1994 Nov 15;315(1):190–198. doi: 10.1006/abbi.1994.1489. [DOI] [PubMed] [Google Scholar]
- Nemoto O., Yamada H., Mukaida M., Shimmei M. Stimulation of TIMP-1 production by oncostatin M in human articular cartilage. Arthritis Rheum. 1996 Apr;39(4):560–566. doi: 10.1002/art.1780390404. [DOI] [PubMed] [Google Scholar]
- Ogata Y., Enghild J. J., Nagase H. Matrix metalloproteinase 3 (stromelysin) activates the precursor for the human matrix metalloproteinase 9. J Biol Chem. 1992 Feb 25;267(6):3581–3584. [PubMed] [Google Scholar]
- Padayatty S. J., Orme S., Zenobi P. D., Stickland M. H., Belchetz P. E., Grant P. J. The effects of insulin-like growth factor-1 on plasminogen activator inhibitor-1 synthesis and secretion: results from in vitro and in vivo studies. Thromb Haemost. 1993 Dec 20;70(6):1009–1013. [PubMed] [Google Scholar]
- Rajah R., Katz L., Nunn S., Solberg P., Beers T., Cohen P. Insulin-like growth factor binding protein (IGFBP) proteases: functional regulators of cell growth. Prog Growth Factor Res. 1995;6(2-4):273–284. doi: 10.1016/0955-2235(95)00012-7. [DOI] [PubMed] [Google Scholar]
- Rogachefsky R. A., Dean D. D., Howell D. S., Altman R. D. Treatment of canine osteoarthritis with sodium pentosan polysulfate and insulin-like growth factor-1. Ann N Y Acad Sci. 1994 Sep 6;732:392–394. doi: 10.1111/j.1749-6632.1994.tb24763.x. [DOI] [PubMed] [Google Scholar]
- Rydziel S., Delany A. M., Canalis E. Insulin-like growth factor I inhibits the transcription of collagenase 3 in osteoblast cultures. J Cell Biochem. 1997 Nov 1;67(2):176–183. [PubMed] [Google Scholar]
- Sah R. L., Chen A. C., Grodzinsky A. J., Trippel S. B. Differential effects of bFGF and IGF-I on matrix metabolism in calf and adult bovine cartilage explants. Arch Biochem Biophys. 1994 Jan;308(1):137–147. doi: 10.1006/abbi.1994.1020. [DOI] [PubMed] [Google Scholar]
- Schouten J. S., Van den Ouweland F. A., Valkenburg H. A., Lamberts S. W. Insulin-like growth factor-1: a prognostic factor of knee osteoarthritis. Br J Rheumatol. 1993 Apr;32(4):274–280. doi: 10.1093/rheumatology/32.4.274. [DOI] [PubMed] [Google Scholar]
- Shlopov B. V., Lie W. R., Mainardi C. L., Cole A. A., Chubinskaya S., Hasty K. A. Osteoarthritic lesions: involvement of three different collagenases. Arthritis Rheum. 1997 Nov;40(11):2065–2074. doi: 10.1002/art.1780401120. [DOI] [PubMed] [Google Scholar]
- Shlopov B. V., Smith G. N., Jr, Cole A. A., Hasty K. A. Differential patterns of response to doxycycline and transforming growth factor beta1 in the down-regulation of collagenases in osteoarthritic and normal human chondrocytes. Arthritis Rheum. 1999 Apr;42(4):719–727. doi: 10.1002/1529-0131(199904)42:4<719::AID-ANR15>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- Su S., Grover J., Roughley P. J., DiBattista J. A., Martel-Pelletier J., Pelletier J. P., Zafarullah M. Expression of the tissue inhibitor of metalloproteinases (TIMP) gene family in normal and osteoarthritic joints. Rheumatol Int. 1999;18(5-6):183–191. doi: 10.1007/s002960050083. [DOI] [PubMed] [Google Scholar]
- Suzuki K., Enghild J. J., Morodomi T., Salvesen G., Nagase H. Mechanisms of activation of tissue procollagenase by matrix metalloproteinase 3 (stromelysin). Biochemistry. 1990 Nov 6;29(44):10261–10270. doi: 10.1021/bi00496a016. [DOI] [PubMed] [Google Scholar]
- Tardif G., Pelletier J. P., Dupuis M., Geng C., Cloutier J. M., Martel-Pelletier J. Collagenase 3 production by human osteoarthritic chondrocytes in response to growth factors and cytokines is a function of the physiologic state of the cells. Arthritis Rheum. 1999 Jun;42(6):1147–1158. doi: 10.1002/1529-0131(199906)42:6<1147::AID-ANR11>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- Tetlow L. C., Woolley D. E. Comparative immunolocalization studies of collagenase 1 and collagenase 3 production in the rheumatoid lesion, and by human chondrocytes and synoviocytes in vitro. Br J Rheumatol. 1998 Jan;37(1):64–70. doi: 10.1093/rheumatology/37.1.64. [DOI] [PubMed] [Google Scholar]
- Tyler J. A. Insulin-like growth factor 1 can decrease degradation and promote synthesis of proteoglycan in cartilage exposed to cytokines. Biochem J. 1989 Jun 1;260(2):543–548. doi: 10.1042/bj2600543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verschure P. J., Van Noorden C. J., Van Marle J., Van den Berg W. B. Articular cartilage destruction in experimental inflammatory arthritis: insulin-like growth factor-1 regulation of proteoglycan metabolism in chondrocytes. Histochem J. 1996 Dec;28(12):835–857. doi: 10.1007/BF02331388. [DOI] [PubMed] [Google Scholar]
- Wu J. J., Lark M. W., Chun L. E., Eyre D. R. Sites of stromelysin cleavage in collagen types II, IX, X, and XI of cartilage. J Biol Chem. 1991 Mar 25;266(9):5625–5628. [PubMed] [Google Scholar]
- Yaeger P. C., Masi T. L., de Ortiz J. L., Binette F., Tubo R., McPherson J. M. Synergistic action of transforming growth factor-beta and insulin-like growth factor-I induces expression of type II collagen and aggrecan genes in adult human articular chondrocytes. Exp Cell Res. 1997 Dec 15;237(2):318–325. doi: 10.1006/excr.1997.3781. [DOI] [PubMed] [Google Scholar]
- van Beuningen H. M., van der Kraan P. M., Arntz O. J., van den Berg W. B. Protection from interleukin 1 induced destruction of articular cartilage by transforming growth factor beta: studies in anatomically intact cartilage in vitro and in vivo. Ann Rheum Dis. 1993 Mar;52(3):185–191. doi: 10.1136/ard.52.3.185. [DOI] [PMC free article] [PubMed] [Google Scholar]