Abstract
OBJECTIVE—To study the efficacy of combination therapy with etanercept and methotrexate in patients with refractory juvenile idiopathic arthritis. METHODS—Seven children with active juvenile idiopathic arthritis refractory to at least combination therapy with methotrexate and sulfasalazine or cyclosporin A were studied. Concomitant treatment, consisting of non-steroidal drugs, corticosteroids, and methotrexate, remained unchanged. RESULTS—Six patients continued the treatment for at least 24 weeks. In the child with systemic arthritis, etanercept was stopped because of persisting spiking fever, joint pain, and rash. In the remaining children an immediate significant decrease in joint pain (p<0.05), disappearance of morning stiffness, and regression of joint swelling (p<0.05) were observed. Improvement was apparent after two injections. An immediate significant (p<0.05) decrease in erythrocyte sedimentation rate, C reactive protein, and interleukin 6 was observed. Side effects consisted of mild reactions at the injection site in two children. CONCLUSIONS—In this observational study, etanercept in combination with methotrexate was well tolerated and highly effective in treating juvenile polyarthritis but not in the patient with systemic arthritis. Combination treatment appears to be feasible in terms of toxicity and may enhance efficiency.
Full Text
The Full Text of this article is available as a PDF (78.5 KB).
Figure 1 .
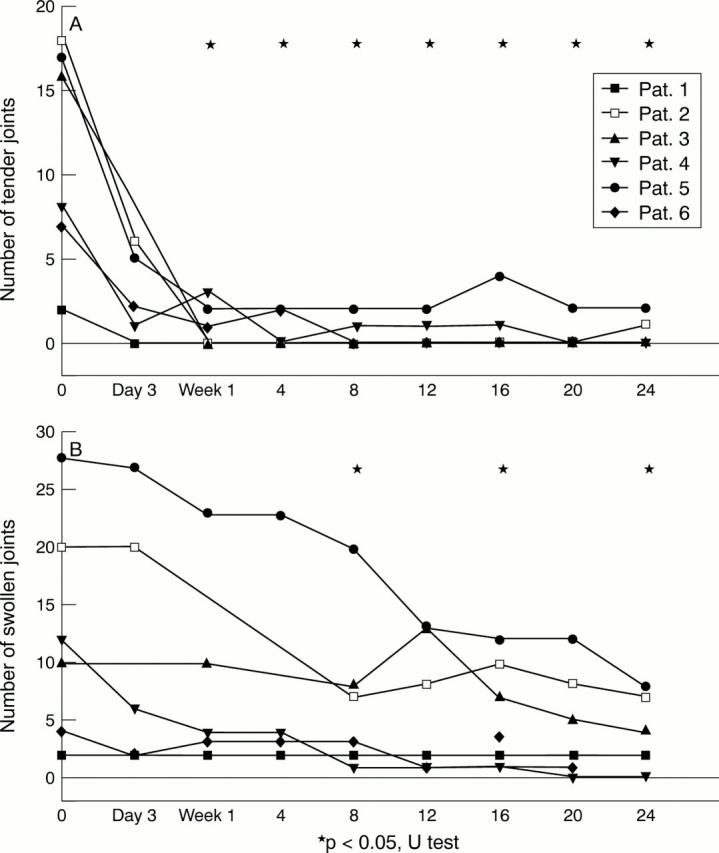
Clinical outcome parameters. (A) The tender joint count and (B) the number of swollen joints decreased significantly (p<0.05, indicated by asterisks). Morning stiffness disappeared in all children after one week of treatment (data not shown).
Figure 2 .
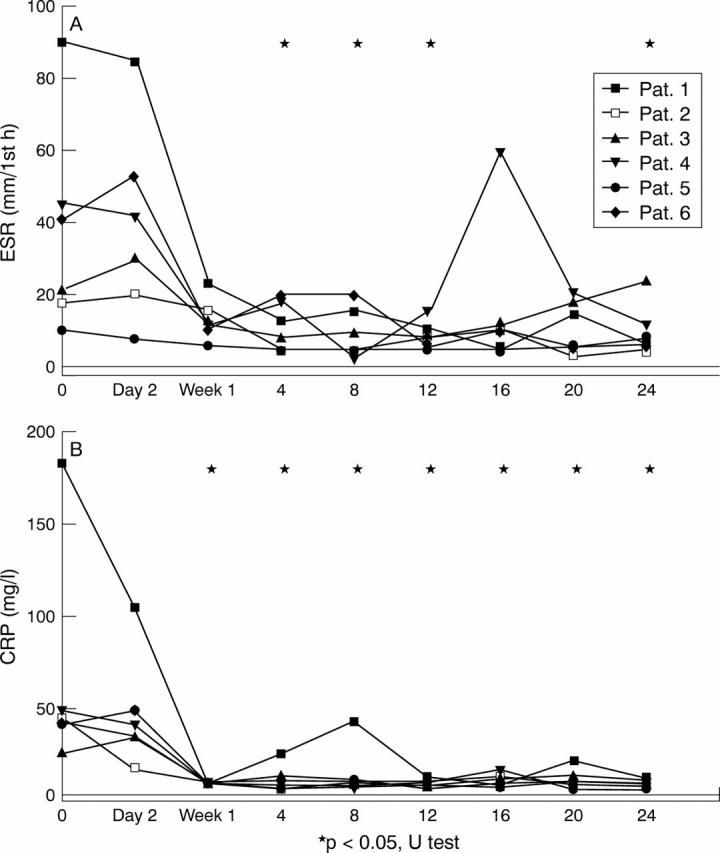
Laboratory outcome parameters. The median erythrocyte sedimentation rate (ESR) decreased significantly from 31 to 8, 7.5, 9, and 12.5 mm/h after one, two, three, and six months respectively (p<0.05, indicated by asterisks). The median C reactive protein (CRP) level significantly decreased from 41.3 to 5.5, 7.1, 6.5, and 6.9 mg/l after one, two, three, and 6 months respectively (p<0.05, indicated by asterisks). The median IL6 level decreased from 34.2 to 6.3 pg/ml, after one month. Thereafter it remained undetectable in sera from five of the six patients (data not shown).


