Abstract
OBJECTIVE—To evaluate the effect of aminoguanidine (AG) on de novo interleukin 1β (IL1β), nitric oxide (NO), and interleukin 1 receptor antagonist (IL1ra) production by osteoarthritic human synovial tissue and articular cartilage cultures. METHODS—Synovial tissue and cartilage, obtained during surgery from 29 patients undergoing total knee or hip replacement for osteoarthritis, were cut into small pieces and cultured in the presence or absence of lipopolysaccharide (LPS) and test materials. IL1β, IL1ra, and NO were determined in culture media. The inducible nitric oxide synthase inhibitor, AG, was added to cultures in various concentrations (0.3-3 mmol/l). RESULTS—In synovial tissue cultures AG (0.3, 1, and 3 mmol/l) decreased LPS (1 µg/ml) stimulated IL1β and NO release in the media in a dose dependent manner (p<0.05 at 1 mmol/l and p<0.05 at 0.3 mmol/l, respectively). In articular cartilage cultures AG (0.3, 1, and 3 mmol/l) decreased LPS (1 µg/ml) stimulated IL1β and NO release in the media in a dose dependent manner (p<0.05 at 1 mmol/l and p<0.01 at 0.3 mmol/l, respectively). Hydrocortisone (5 µg/ml) also significantly decreased LPS stimulated IL1β release in media of synovial tissue and cartilage cultures and NO in media of synovial cultures. AG (0.3, 1, and 3 mmol/l) decreased LPS (1 µg/ml) stimulated IL1ra levels in media of synovial tissue cultures in a dose dependent manner (p<0.05 at 1 mmol/l) but increased LPS (1 µg/ml) stimulated IL1ra release in media of cartilage cultures (p<0.01 at 3 mmol/l). The NO donor, nitroprusside (10, 30, 100, and 300 µg/ml) stimulated IL1β release in media of synovial tissue cultures in a dose dependent manner (p<0.01 at 100 µg/ml). AG and nitroprusside at the concentrations used had no toxic effect on human synovial cells. CONCLUSIONS—NO synthase inhibitors may modulate osteoarthritis and articular inflammatory processes not only by decreasing NO synthesis but also by their effects on ILβ and IL1ra production.
Full Text
The Full Text of this article is available as a PDF (85.6 KB).
Figure 1 .
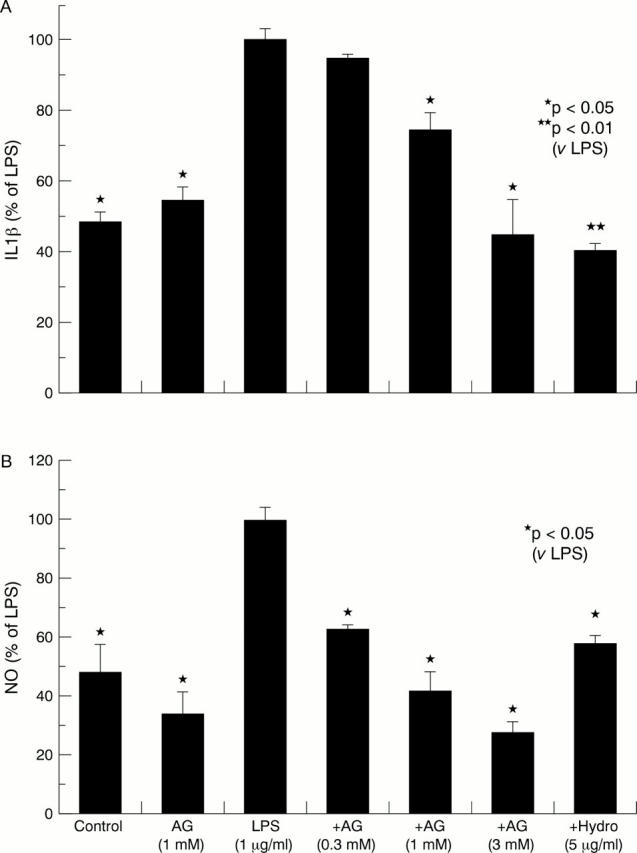
Effect of aminoguanidine (AG) and hydrocortisone (Hydro) on the release of interleukin 1β (IL1β) and nitric oxide (NO) in the media of lipopolysaccharide (LPS) stimulated synovial tissue cultures. Bars show the mean and SEM of three separate experiments. The absolute mean (SEM) levels of IL1β in control and in LPS stimulated cultures were 7.3 (0.89) and 14.9 (2.5) pg/mg synovia, respectively. The absolute mean (SEM) levels of NO in control and LPS stimulated cultures were 2.13 (0.2) and 4.34 (0.19) µmol/mg synovial tissue, respectively.
Figure 2 .
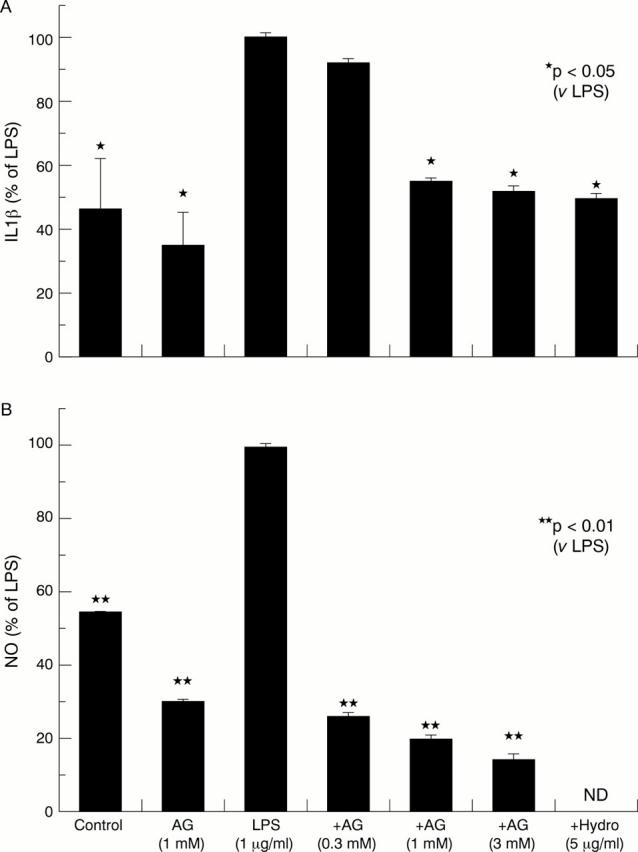
Effect of aminoguanidine (AG) on the release of interleukin 1β (IL1β) and nitric oxide (NO) in the media of lipopolysaccharide (LPS) stimulated cartilage cultures. Bars show the mean and SEM of six separate experiments. The absolute mean (SEM) levels of IL1β in control and LPS stimulated cultures were 0.29 (0.01) and 0.64 (0.12) pg/mg cartilage, respectively. The absolute mean (SEM) levels of NO in control and LPS stimulated cultures were 15.8 (0.001) and 30.68 (0.003) µmol/mg cartilage, respectively.
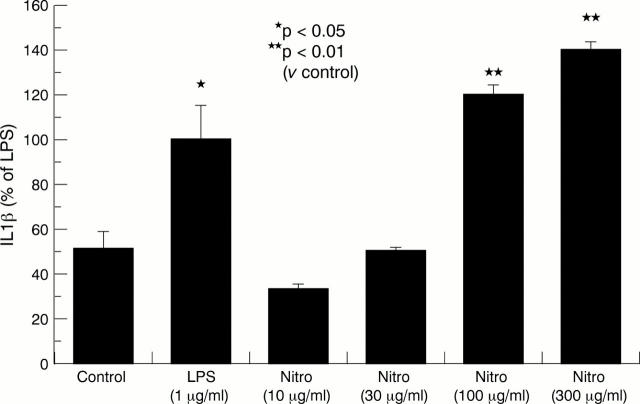
Figure 3 .
Effect of nitroprusside (Nitro) on interleukin 1β (IL1β) release in media of synovial tissue cultures. Bars show the mean and SEM of three separate experiments. The absolute mean (SEM) levels of IL1β in control and lipopolysaccharide (LPS) stimulated cultures were 7.46 (3.69) and 13.69 (4.5) pg/mg synovia, respectively.
Figure 4 .
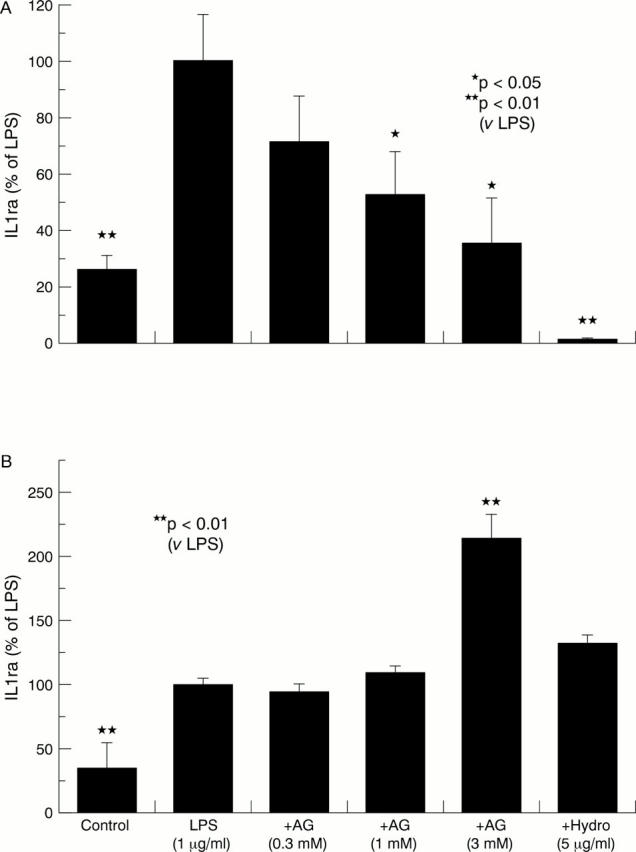
Effect of aminoguanidine (AG) and hydrocortisone (Hydro) on the release of interleukin 1 receptor antagonist (IL1ra) in lipopolysaccharide (LPS) stimulated synovial tissue (A) and cartilage (B) cultures. Bars show the mean and SEM of three separate experiments. The absolute mean (SEM) levels of IL1ra in control and LPS stimulated cultures were 163.5 (29.84) and 651 (107) pg/mg synovia, respectively, and for cartilage cultures 1.4 (0.2) and 4.5 (0.24) pg/mg cartilage, respectively.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amin A. R., Abramson S. B. The role of nitric oxide in articular cartilage breakdown in osteoarthritis. Curr Opin Rheumatol. 1998 May;10(3):263–268. doi: 10.1097/00002281-199805000-00018. [DOI] [PubMed] [Google Scholar]
- Ashab I., Peer G., Blum M., Wollman Y., Chernihovsky T., Hassner A., Schwartz D., Cabili S., Silverberg D., Iaina A. Oral administration of L-arginine and captopril in rats prevents chronic renal failure by nitric oxide production. Kidney Int. 1995 Jun;47(6):1515–1521. doi: 10.1038/ki.1995.214. [DOI] [PubMed] [Google Scholar]
- Di Genaro M. S., Muñoz E., Aguilera C., de Guzmán A. M. Yersinia enterocolitica O:8 and O:5 lipopolysaccharide arthritogenicity in hamsters. Rheumatology (Oxford) 2000 Jan;39(1):73–78. doi: 10.1093/rheumatology/39.1.73. [DOI] [PubMed] [Google Scholar]
- Evans C. H. Nitric oxide: what role does it play in inflammation and tissue destruction? Agents Actions Suppl. 1995;47:107–116. doi: 10.1007/978-3-0348-7343-7_9. [DOI] [PubMed] [Google Scholar]
- Evans CH, Stefanovic-Racic M. Nitric Oxide in Arthritis. Methods. 1996 Aug;10(1):38–42. doi: 10.1006/meth.1996.0076. [DOI] [PubMed] [Google Scholar]
- Häuselmann H. J., Stefanovic-Racic M., Michel B. A., Evans C. H. Differences in nitric oxide production by superficial and deep human articular chondrocytes: implications for proteoglycan turnover in inflammatory joint diseases. J Immunol. 1998 Feb 1;160(3):1444–1448. [PubMed] [Google Scholar]
- Kostic T. S., Andric S. A., Maric D., Stojilkovic S. S., Kovacevic R. Involvement of inducible nitric oxide synthase in stress-impaired testicular steroidogenesis. J Endocrinol. 1999 Dec;163(3):409–416. doi: 10.1677/joe.0.1630409. [DOI] [PubMed] [Google Scholar]
- Martel-Pelletier J., Mineau F., Jolicoeur F. C., Cloutier J. M., Pelletier J. P. In vitro effects of diacerhein and rhein on interleukin 1 and tumor necrosis factor-alpha systems in human osteoarthritic synovium and chondrocytes. J Rheumatol. 1998 Apr;25(4):753–762. [PubMed] [Google Scholar]
- McInnes I. B., Leung B. P., Field M., Wei X. Q., Huang F. P., Sturrock R. D., Kinninmonth A., Weidner J., Mumford R., Liew F. Y. Production of nitric oxide in the synovial membrane of rheumatoid and osteoarthritis patients. J Exp Med. 1996 Oct 1;184(4):1519–1524. doi: 10.1084/jem.184.4.1519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Misko T. P., Moore W. M., Kasten T. P., Nickols G. A., Corbett J. A., Tilton R. G., McDaniel M. L., Williamson J. R., Currie M. G. Selective inhibition of the inducible nitric oxide synthase by aminoguanidine. Eur J Pharmacol. 1993 Mar 16;233(1):119–125. doi: 10.1016/0014-2999(93)90357-n. [DOI] [PubMed] [Google Scholar]
- Murrell G. A., Jang D., Williams R. J. Nitric oxide activates metalloprotease enzymes in articular cartilage. Biochem Biophys Res Commun. 1995 Jan 5;206(1):15–21. doi: 10.1006/bbrc.1995.1003. [DOI] [PubMed] [Google Scholar]
- Müller-Ladner U., Roberts C. R., Franklin B. N., Gay R. E., Robbins P. D., Evans C. H., Gay S. Human IL-1Ra gene transfer into human synovial fibroblasts is chondroprotective. J Immunol. 1997 Apr 1;158(7):3492–3498. [PubMed] [Google Scholar]
- Pelletier J. P., Jovanovic D., Fernandes J. C., Manning P., Connor J. R., Currie M. G., Di Battista J. A., Martel-Pelletier J. Reduced progression of experimental osteoarthritis in vivo by selective inhibition of inducible nitric oxide synthase. Arthritis Rheum. 1998 Jul;41(7):1275–1286. doi: 10.1002/1529-0131(199807)41:7<1275::AID-ART19>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- Pelletier J. P., Mineau F., Ranger P., Tardif G., Martel-Pelletier J. The increased synthesis of inducible nitric oxide inhibits IL-1ra synthesis by human articular chondrocytes: possible role in osteoarthritic cartilage degradation. Osteoarthritis Cartilage. 1996 Mar;4(1):77–84. doi: 10.1016/s1063-4584(96)80009-4. [DOI] [PubMed] [Google Scholar]
- Roehm N. W., Rodgers G. H., Hatfield S. M., Glasebrook A. L. An improved colorimetric assay for cell proliferation and viability utilizing the tetrazolium salt XTT. J Immunol Methods. 1991 Sep 13;142(2):257–265. doi: 10.1016/0022-1759(91)90114-u. [DOI] [PubMed] [Google Scholar]
- St Clair E. W. Nitric oxide--friend or foe in arthritis? J Rheumatol. 1998 Aug;25(8):1451–1453. [PubMed] [Google Scholar]
- Stefanovic-Racic M., Meyers K., Meschter C., Coffey J. W., Hoffman R. A., Evans C. H. Comparison of the nitric oxide synthase inhibitors methylarginine and aminoguanidine as prophylactic and therapeutic agents in rat adjuvant arthritis. J Rheumatol. 1995 Oct;22(10):1922–1928. [PubMed] [Google Scholar]
- Stefanovic-Racic M., Möllers M. O., Miller L. A., Evans C. H. Nitric oxide and proteoglycan turnover in rabbit articular cartilage. J Orthop Res. 1997 May;15(3):442–449. doi: 10.1002/jor.1100150318. [DOI] [PubMed] [Google Scholar]
- Stefanovic-Racic M., Stadler J., Georgescu H. I., Evans C. H. Nitric oxide synthesis and its regulation by rabbit synoviocytes. J Rheumatol. 1994 Oct;21(10):1892–1898. [PubMed] [Google Scholar]
- Studer R., Jaffurs D., Stefanovic-Racic M., Robbins P. D., Evans C. H. Nitric oxide in osteoarthritis. Osteoarthritis Cartilage. 1999 Jul;7(4):377–379. doi: 10.1053/joca.1998.0216. [DOI] [PubMed] [Google Scholar]
- Taskiran D., Stefanovic-Racic M., Georgescu H., Evans C. Nitric oxide mediates suppression of cartilage proteoglycan synthesis by interleukin-1. Biochem Biophys Res Commun. 1994 Apr 15;200(1):142–148. doi: 10.1006/bbrc.1994.1426. [DOI] [PubMed] [Google Scholar]
- Van de Loo F. A., Arntz O. J., Van den Berg W. B. Effect of interleukin 1 and leukaemia inhibitory factor on chondrocyte metabolism in articular cartilage from normal and interleukin-6-deficient mice: role of nitric oxide and IL-6 in the suppression of proteoglycan synthesis. Cytokine. 1997 Jul;9(7):453–462. doi: 10.1006/cyto.1997.0188. [DOI] [PubMed] [Google Scholar]
- Yaron I., Meyer F. A., Dayer J. M., Bleiberg I., Yaron M. Some recombinant human cytokines stimulate glycosaminoglycan synthesis in human synovial fibroblast cultures and inhibit it in human articular cartilage cultures. Arthritis Rheum. 1989 Feb;32(2):173–180. doi: 10.1002/anr.1780320210. [DOI] [PubMed] [Google Scholar]
- Yaron I., Shirazi I., Judovich R., Levartovsky D., Caspi D., Yaron M. Fluoxetine and amitriptyline inhibit nitric oxide, prostaglandin E2, and hyaluronic acid production in human synovial cells and synovial tissue cultures. Arthritis Rheum. 1999 Dec;42(12):2561–2568. doi: 10.1002/1529-0131(199912)42:12<2561::AID-ANR8>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- Yaron M., Shirazi I., Yaron I. Anti-interleukin-1 effects of diacerein and rhein in human osteoarthritic synovial tissue and cartilage cultures. Osteoarthritis Cartilage. 1999 May;7(3):272–280. doi: 10.1053/joca.1998.0201. [DOI] [PubMed] [Google Scholar]
- de Mello S. B., Novaes G. S., Laurindo I. M., Muscará M. N., Maciel F. M., Cossermelli W. Nitric oxide synthase inhibitor influences prostaglandin and interleukin-1 production in experimental arthritic joints. Inflamm Res. 1997 Feb;46(2):72–77. doi: 10.1007/s000110050086. [DOI] [PubMed] [Google Scholar]
- van den Berg W. B. Lessons for joint destruction from animal models. Curr Opin Rheumatol. 1997 May;9(3):221–228. doi: 10.1097/00002281-199705000-00008. [DOI] [PubMed] [Google Scholar]
- van den Berg W. B. The role of cytokines and growth factors in cartilage destruction in osteoarthritis and rheumatoid arthritis. Z Rheumatol. 1999 Jun;58(3):136–141. doi: 10.1007/s003930050163. [DOI] [PubMed] [Google Scholar]