Abstract
OBJECTIVE—To compare the activity of calcineurin in the peripheral blood mononuclear cells (PBMC) of 32 patients with systemic lupus erythematosus (SLE) and 35 healthy controls. METHODS—The activity of calcineurin was assayed in the supernatants of sonicated mononuclear cells. RESULTS—There was no significant difference in the calcineurin activity of patients with SLE not taking glucocorticosteroids (GCS) compared with the healthy controls. On the other hand, the activity of calcineurin was reduced in patients with SLE taking GCS, correlating negatively with the dose of GCS. The stimulation of PBMC by phorbol ester and calcium ionophore decreased the calcineurin activity both in patients with SLE and in healthy controls. GCS could also reduce calcineurin activity in the mononuclear cells of healthy subjects in vitro. CONCLUSIONS—In patients with SLE the decrease in the calcineurin activity of PBMC depended on the dose of GCS used for treatment, and it was not a disease specific alteration. The higher the dose of GCS, the greater the inhibition of calcineurin activity. The reduction of calcineurin activity is a new element in the immunosuppressive effects of GCS during the treatment of patients with SLE.
Full Text
The Full Text of this article is available as a PDF (88.1 KB).
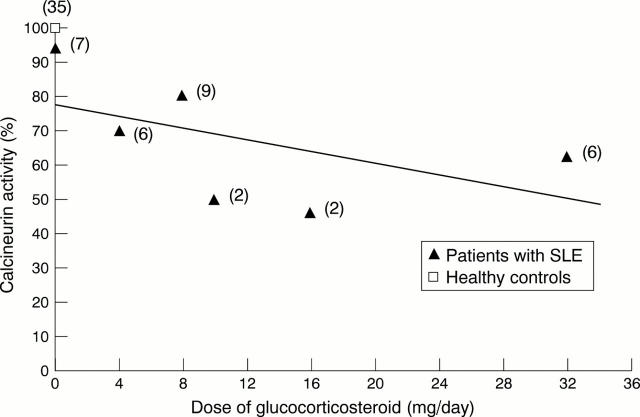
Figure 1 .
Negative correlation between the basal activities of calcineurin in the peripheral blood mononuclear cells (PBMC) of patients with systemic lupus erythematosus (SLE) and the doses of glucocorticosteroids (GCS) used for treatment. The coefficient of correlation between the basal calcineurin activities in the PBMC and the daily doses of GCS (0-32 mg/day) taken by patients with SLE was calculated. The calcineurin activity of healthy controls were taken as 100%. The number of patients is given in parentheses.
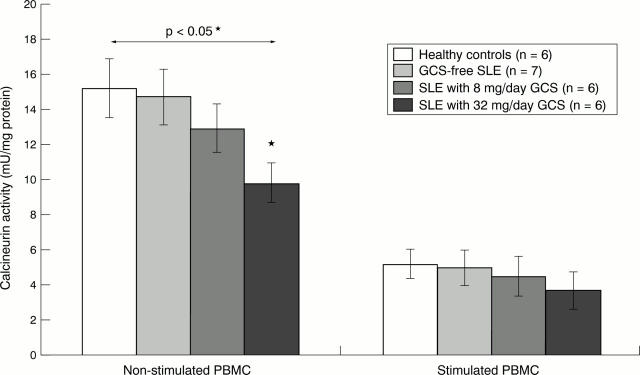
Figure 2 .
Calcineurin activities in the peripheral blood mononuclear cells (PBMC) of patients with systemic lupus erythematosus (SLE) with or without glucocorticosteroid (GCS) treatment and in healthy controls. Calcineurin activities were assayed in the supernatants of non-stimulated and stimulated PBMC as described in "Patients and methods". The statistical significance of the differences was calculated by Student's unpaired t test. Asterisk denotes significant difference compared with the controls.
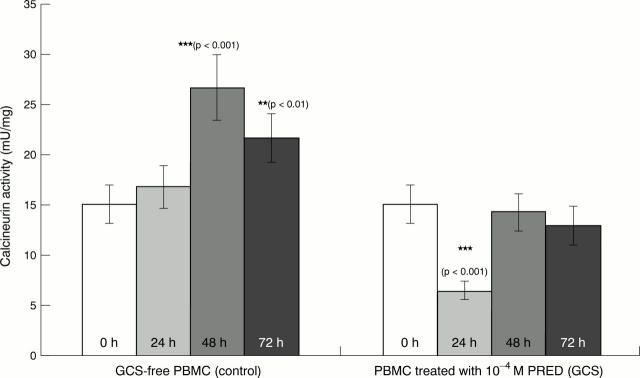
Figure 3 .
In vitro effect of prednisolone sodium succinate (PRED) on the activity of calcineurin in peripheral blood mononuclear cells (PBMC) of healthy subjects. PBMC of five healthy controls were cultured for 72 hours in the absence and presence of 10−4 M PRED. Calcineurin activities were determined and the statistical analysis was carried out as described in "Patients and methods". Asterisks denote significant differences compared with the respective controls. For the calculation of statistical significance Student's paired t test was used.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barnes P. J. Molecular mechanisms of steroid action in asthma. J Allergy Clin Immunol. 1996 Jan;97(1 Pt 2):159–168. doi: 10.1016/s0091-6749(96)80216-8. [DOI] [PubMed] [Google Scholar]
- Bing R. J., Dudek R., Kähler J., Narayan K. S., Ingram M. Cytokine production from freshly harvested human mononuclear cells attached to plastic beads. Tissue Cell. 1992;24(2):203–209. doi: 10.1016/0040-8166(92)90093-m. [DOI] [PubMed] [Google Scholar]
- Bombardier C., Gladman D. D., Urowitz M. B., Caron D., Chang C. H. Derivation of the SLEDAI. A disease activity index for lupus patients. The Committee on Prognosis Studies in SLE. Arthritis Rheum. 1992 Jun;35(6):630–640. doi: 10.1002/art.1780350606. [DOI] [PubMed] [Google Scholar]
- Buttgereit F., Brand M. D., Burmester G. R. Equivalent doses and relative drug potencies for non-genomic glucocorticoid effects: a novel glucocorticoid hierarchy. Biochem Pharmacol. 1999 Jul 15;58(2):363–368. doi: 10.1016/s0006-2952(99)00090-8. [DOI] [PubMed] [Google Scholar]
- Buttgereit F., Wehling M., Burmester G. R. A new hypothesis of modular glucocorticoid actions: steroid treatment of rheumatic diseases revisited. Arthritis Rheum. 1998 May;41(5):761–767. doi: 10.1002/1529-0131(199805)41:5<761::AID-ART2>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- Böyum A. Isolation of mononuclear cells and granulocytes from human blood. Isolation of monuclear cells by one centrifugation, and of granulocytes by combining centrifugation and sedimentation at 1 g. Scand J Clin Lab Invest Suppl. 1968;97:77–89. [PubMed] [Google Scholar]
- Chen R., Burke T. F., Cumberland J. E., Brummet M., Beck L. A., Casolaro V., Georas S. N. Glucocorticoids inhibit calcium- and calcineurin-dependent activation of the human IL-4 promoter. J Immunol. 2000 Jan 15;164(2):825–832. doi: 10.4049/jimmunol.164.2.825. [DOI] [PubMed] [Google Scholar]
- Cohen P., Foulkes J. G., Holmes C. F., Nimmo G. A., Tonks N. K. Protein phosphatase inhibitor-1 and inhibitor-2 from rabbit skeletal muscle. Methods Enzymol. 1988;159:427–437. doi: 10.1016/0076-6879(88)59042-0. [DOI] [PubMed] [Google Scholar]
- Guerini D. Calcineurin: not just a simple protein phosphatase. Biochem Biophys Res Commun. 1997 Jun 18;235(2):271–275. doi: 10.1006/bbrc.1997.6802. [DOI] [PubMed] [Google Scholar]
- Gyimesi E., Kavai M., Kiss E., Csipö I., Szücs G., Szegedi G. Triggering of respiratory burst by phagocytosis in monocytes of patients with systemic lupus erythematosus. Clin Exp Immunol. 1993 Oct;94(1):140–144. doi: 10.1111/j.1365-2249.1993.tb05991.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hochberg M. C. Updating the American College of Rheumatology revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum. 1997 Sep;40(9):1725–1725. doi: 10.1002/art.1780400928. [DOI] [PubMed] [Google Scholar]
- Kammer G. M. High prevalence of T cell type I protein kinase A deficiency in systemic lupus erythematosus. Arthritis Rheum. 1999 Jul;42(7):1458–1465. doi: 10.1002/1529-0131(199907)42:7<1458::AID-ANR20>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- Laxminarayana D., Khan I. U., Mishra N., Olorenshaw I., Taskén K., Kammer G. M. Diminished levels of protein kinase A RI alpha and RI beta transcripts and proteins in systemic lupus erythematosus T lymphocytes. J Immunol. 1999 May 1;162(9):5639–5648. [PubMed] [Google Scholar]
- Lipworth B. J. Therapeutic implications of non-genomic glucocorticoid activity. Lancet. 2000 Jul 8;356(9224):87–89. doi: 10.1016/S0140-6736(00)02463-6. [DOI] [PubMed] [Google Scholar]
- Menè P., Pecci G., Cinotti G. A., Pugliese G., Pricci F., Pugliese F. Eicosanoid synthesis in peripheral blood monocytes: a marker of disease activity in lupus nephritis. Am J Kidney Dis. 1998 Nov;32(5):778–784. doi: 10.1016/s0272-6386(98)70133-7. [DOI] [PubMed] [Google Scholar]
- Paliogianni F., Boumpas D. T. Glucocorticoids regulate calcineurin-dependent trans-activating pathways for interleukin-2 gene transcription in human T lymphocytes. Transplantation. 1995 May 15;59(9):1333–1339. [PubMed] [Google Scholar]
- Phi N. C., Takáts A., Binh V. H., Vien C. V., González-Cabello R., Gergely P. Cyclic AMP level of lymphocytes in patients with systemic lupus erythematosus and its relation to disease activity. Immunol Lett. 1989 Nov;23(1):61–64. doi: 10.1016/0165-2478(89)90156-9. [DOI] [PubMed] [Google Scholar]
- Phillips R., Lomnitzer R., Wadee A. A., Rabson A. R. Defective monocyte function in patients with systemic lupus erythematosus. Clin Immunol Immunopathol. 1985 Jan;34(1):69–76. doi: 10.1016/0090-1229(85)90008-x. [DOI] [PubMed] [Google Scholar]
- Rider V., Foster R. T., Evans M., Suenaga R., Abdou N. I. Gender differences in autoimmune diseases: estrogen increases calcineurin expression in systemic lupus erythematosus. Clin Immunol Immunopathol. 1998 Nov;89(2):171–180. doi: 10.1006/clin.1998.4604. [DOI] [PubMed] [Google Scholar]
- Rider V., Jones S. R., Evans M., Abdou N. I. Molecular mechanisms involved in the estrogen-dependent regulation of calcineurin in systemic lupus erythematosus T cells. Clin Immunol. 2000 May;95(2):124–134. doi: 10.1006/clim.2000.4844. [DOI] [PubMed] [Google Scholar]
- Rühlmann A., Nordheim A. Effects of the immunosuppressive drugs CsA and FK506 on intracellular signalling and gene regulation. Immunobiology. 1997 Dec;198(1-3):192–206. doi: 10.1016/S0171-2985(97)80040-X. [DOI] [PubMed] [Google Scholar]
- Seki M., Ushiyama C., Seta N., Abe K., Fukazawa T., Asakawa J., Takasaki Y., Hashimoto H. Apoptosis of lymphocytes induced by glucocorticoids and relationship to therapeutic efficacy in patients with systemic lupus erythematosus. Arthritis Rheum. 1998 May;41(5):823–830. doi: 10.1002/1529-0131(199805)41:5<823::AID-ART8>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- Shome G. P., Yamane K. Decreased release of leukotriene B4 from monocytes and polymorphonuclear leukocytes in patients with systemic lupus erythematosus. Arerugi. 1991 Jan;40(1):72–81. [PubMed] [Google Scholar]
- Sipka S., Szücs K., Szántó S., Kovács I., Lakos G., Antal-Szalmás P., Szegedi G., Gergely P. Inhibition of calcineurin activity and protection against cyclosporine A induced cytotoxicity by prednisolone sodium succinate in human peripheral mononuclear cells. Immunopharmacology. 2000 Jun;48(1):87–92. doi: 10.1016/s0162-3109(00)00180-6. [DOI] [PubMed] [Google Scholar]
- Sun L., Youn H. D., Loh C., Stolow M., He W., Liu J. O. Cabin 1, a negative regulator for calcineurin signaling in T lymphocytes. Immunity. 1998 Jun;8(6):703–711. doi: 10.1016/s1074-7613(00)80575-0. [DOI] [PubMed] [Google Scholar]
- Tada Y., Nagasawa K., Yamauchi Y., Tsukamoto H., Niho Y. A defect in the protein kinase C system in T cells from patients with systemic lupus erythematosus. Clin Immunol Immunopathol. 1991 Aug;60(2):220–231. doi: 10.1016/0090-1229(91)90065-i. [DOI] [PubMed] [Google Scholar]
- Tsokos G. C., Liossis S. N. Immune cell signaling defects in lupus: activation, anergy and death. Immunol Today. 1999 Mar;20(3):119–124. doi: 10.1016/s0167-5699(98)01395-4. [DOI] [PubMed] [Google Scholar]
- Vázquez-Doval J., Sánchez-Ibarrola A. Defective mononuclear phagocyte function in systemic lupus erythematosus: relationship of FcRII (CD32) with intermediate cytoskeletal filaments. J Investig Allergol Clin Immunol. 1993 Mar-Apr;3(2):86–91. [PubMed] [Google Scholar]
- Wilkinson M. F., Earle M. L., Triggle C. R., Barnes S. Interleukin-1beta, tumor necrosis factor-alpha, and LPS enhance calcium channel current in isolated vascular smooth muscle cells of rat tail artery. FASEB J. 1996 May;10(7):785–791. doi: 10.1096/fasebj.10.7.8635696. [DOI] [PubMed] [Google Scholar]
- Wong H. K., Kammer G. M., Dennis G., Tsokos G. C. Abnormal NF-kappa B activity in T lymphocytes from patients with systemic lupus erythematosus is associated with decreased p65-RelA protein expression. J Immunol. 1999 Aug 1;163(3):1682–1689. [PubMed] [Google Scholar]
- Yang S. D., Tallant E. A., Cheung W. Y. Calcineurin is a calmodulin-dependent protein phosphatase. Biochem Biophys Res Commun. 1982 Jun 30;106(4):1419–1425. doi: 10.1016/0006-291x(82)91272-4. [DOI] [PubMed] [Google Scholar]
- Youn H. D., Grozinger C. M., Liu J. O. Calcium regulates transcriptional repression of myocyte enhancer factor 2 by histone deacetylase 4. J Biol Chem. 2000 Jul 21;275(29):22563–22567. doi: 10.1074/jbc.C000304200. [DOI] [PubMed] [Google Scholar]
- Youn H. D., Sun L., Prywes R., Liu J. O. Apoptosis of T cells mediated by Ca2+-induced release of the transcription factor MEF2. Science. 1999 Oct 22;286(5440):790–793. doi: 10.1126/science.286.5440.790. [DOI] [PubMed] [Google Scholar]
- Zhao Y., Tozawa Y., Iseki R., Mukai M., Iwata M. Calcineurin activation protects T cells from glucocorticoid-induced apoptosis. J Immunol. 1995 Jun 15;154(12):6346–6354. [PubMed] [Google Scholar]