Abstract
OBJECTIVE—To determine the circulating levels of nerve growth factor (NGF), neuropeptide Y (NPY), and vasoactive intestinal peptide (VIP) in systemic sclerosis (SSc), and to correlate these levels with clinical and laboratory features. METHODS—Forty four patients with SSc were evaluated for circulating NGF (immunoenzymatic assay), NPY and VIP (radioimmunoassay), anticentromere and antitopoisomerase I autoantibodies, lung disease (pulmonary function tests with carbon monoxide transfer factor (TLCO), ventilation scintiscan with 99mTc DTPA radioaerosol, high resolution computed tomography (HRCT), pulmonary pressure (echo colour Doppler)), heart disease (standard and 24 ECG, echocardiography), cutaneous involvement (skin score), joint involvement (evidence of tender or swollen joints, or both), peripheral nervous system (PNS) involvement (electromyography), rheumatoid factor, angiotensin converting enzyme (fluorimetric method), von Willebrand factor (ELISA), and erythrocyte sedimentation rate (ESR) (Westergren). RESULTS—Circulating NGF levels in SSc were significantly increased compared with controls (p<0.00001) and significantly higher in the diffuse than in the limited subset of patients (p<0.01). Patients with articular disease had significantly higher levels of NGF. A significant indirect correlation between NGF levels and TLCO was detected (p<0.01), but no correlation was found between NGF and HRCT, DTPA, skin score, PNS involvement and angiotensin converting enzyme and von Willebrand factor levels, antitopoisomerase or anticentromere antibodies, and ESR. NGF levels increased progressively as the disease worsened. Similarly, VIP circulating levels were significantly increased in patients with SSc (p<0.001), whereas the increase of NPY levels did not reach statistical significance. However, both neuropeptides, following the same trend as NGF, increased as the disease worsened (skin score and lung disease). CONCLUSIONS—The increase of NGF and VIP in patients with SSc, the former in the diffuse subset of the disease, and in patients with prominent articular disease, may suggest a link between neurotransmitters and the disease pathogenesis. Neuropeptide circulating levels seem to increase only in patients with the most severe disease.
Full Text
The Full Text of this article is available as a PDF (173.9 KB).
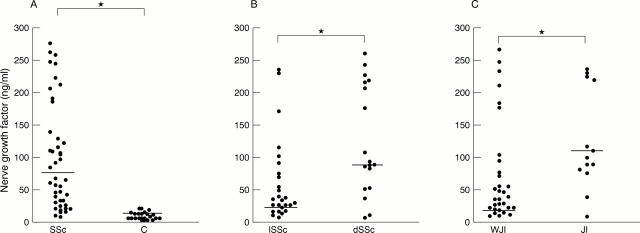
Figure 1 .
(A) Serum levels of nerve growth factor (NGF) (ng/ml) in patients with systemic sclerosis (SSc) and controls (C),*p<0.00001; (B) NGF levels (ng/ml) in limited (lSSc) and diffuse (dSSc) subsets of patients with SSc, *p<0.01; (C) NGF levels (ng/ml) in patients with SSc with joint involvement (JI) and without joint involvement (WJI), *p<0.00002.
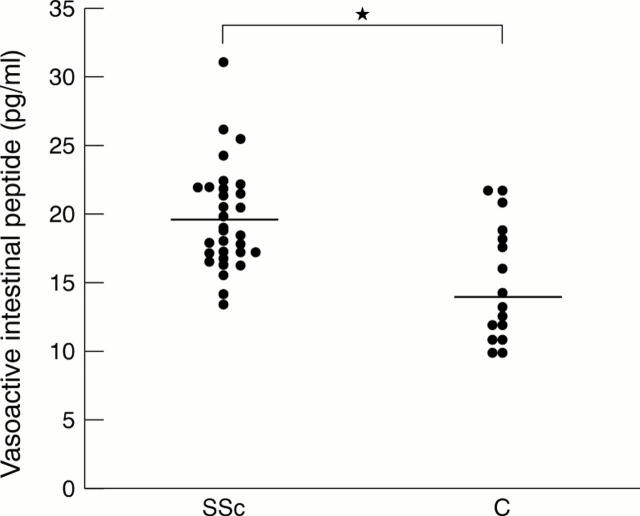
Figure 2 .
Vasoactive intestinal peptide levels (pg/ml) in the sera of patients with systemic sclerosis (SSc) and controls (C), p<0.001.
Figure 3 .
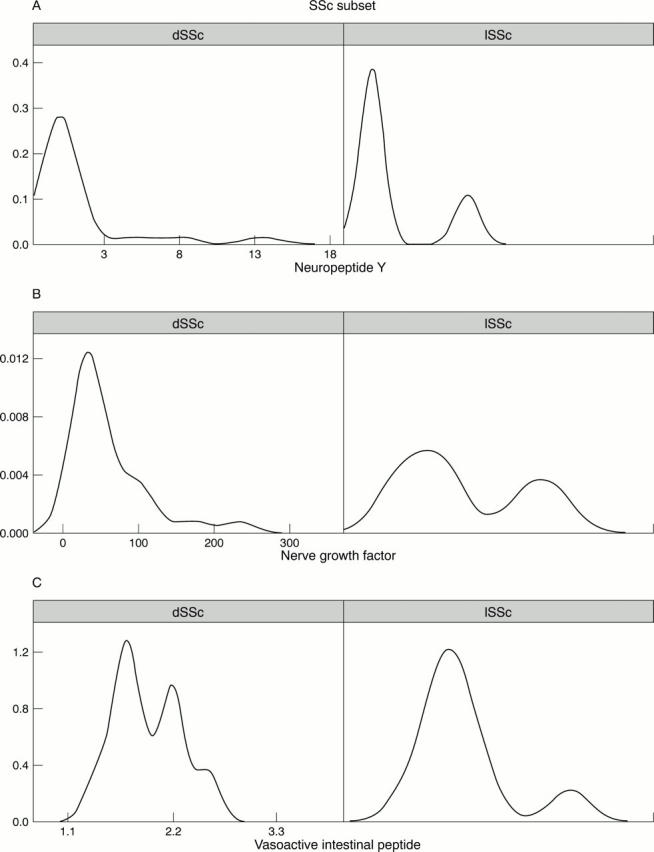
Data mining elaborations visualised by Trellis graphics (showing relations between different variables in a dataset through a conditioning operation). In the three series of two panels x-y plots are reported, conditioned by a chosen variable. In fig 3A, B, and C are represented, respectively, the conditioning of the two systemic sclerosis (SSc) subsets (diffuse subset (dSSc) and limited subset of the disease (lSSc)) (the range is shown in the x axis, the probability density is shown in y-axis) by neuropeptide Y (NPY), nerve growth factor (NGF), and vasoactive intestinal peptide (VIP). Higher NGF and NPY levels are clustered in patients with the diffuse subset compared with those with the limited subset of the disease.
Figure 4 .
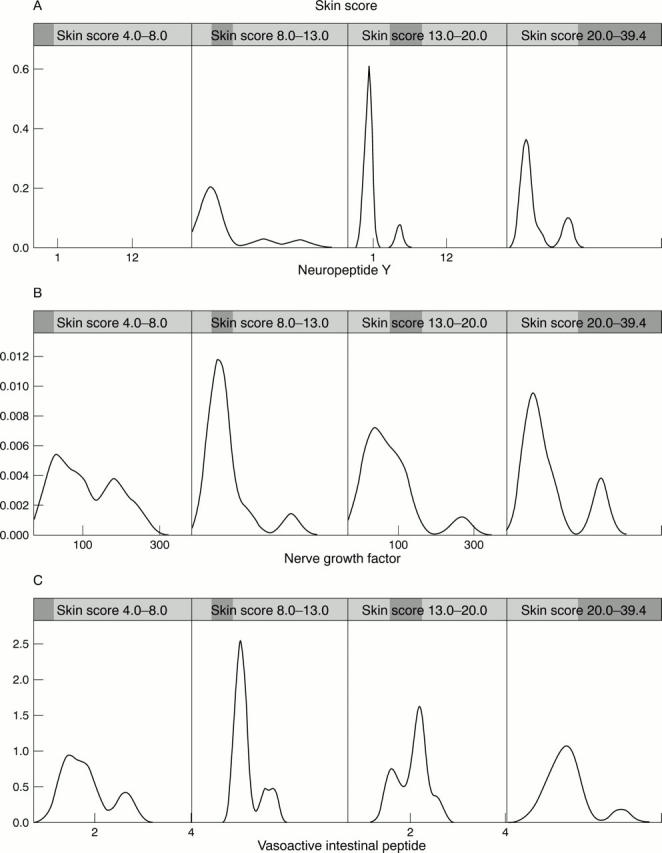
Data mining elaborations represented by Trellis graphics. In A, B, and C are shown, respectively, the conditioning of the the skin score (the range is shown in the x axis, the probability density is shown in the y axis) by neuropeptide Y (NPY), nerve growth factor (NGF), and vasoactive intestinal peptide (VIP). As the skin score increases, a cluster of patients with higher levels of NGF (A) and NPY (B) is identified. This trend is less evident for VIP (C).
Figure 5 .
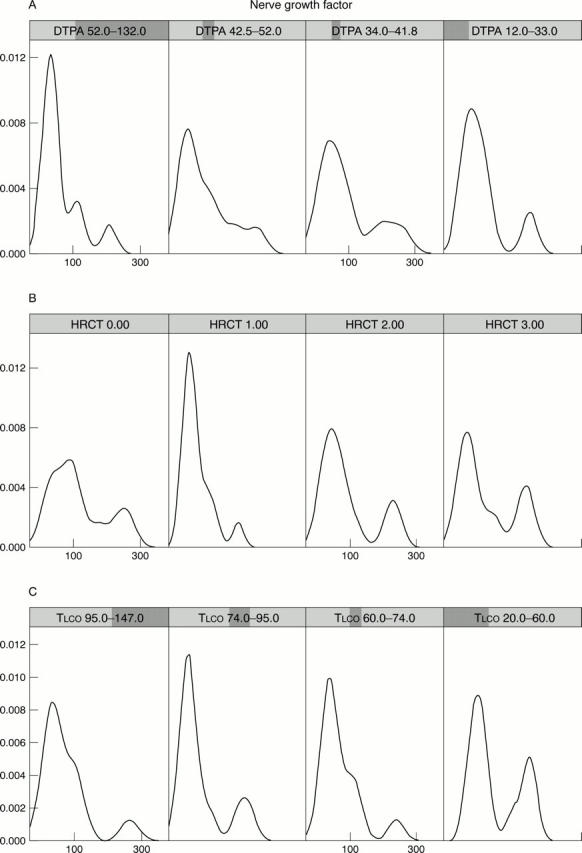
Data mining elaborations represented by Trellis graphics. The involvement of the lung is evaluated with the carbon monoxide transfer factor (TLCO), high resolution computed tomography (HRCT), and DTPA. The sequence of four panels reported in A, B, and C, respectively, shows the conditioning of the NGF density (x axis NGF range, y axis NGF probability density) by different DTPA, HRCT, and TLCO ranges. The parameters showing a worsening of the lung function (increase of HRCT score, decrease of TLCO and DTPA) form a cluster of the group (with diffuse disease) with the highest levels of NGF, NPY, and VIP.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abe T., Morgan D. A., Gutterman D. D. Protective role of nerve growth factor against postischemic dysfunction of sympathetic coronary innervation. Circulation. 1997 Jan 7;95(1):213–220. doi: 10.1161/01.cir.95.1.213. [DOI] [PubMed] [Google Scholar]
- Aloe L., Skaper S. D., Leon A., Levi-Montalcini R. Nerve growth factor and autoimmune diseases. Autoimmunity. 1994;19(2):141–150. doi: 10.3109/08916939409009542. [DOI] [PubMed] [Google Scholar]
- Aloe L., Tuveri M. A. Nerve growth factor and autoimmune rheumatic diseases. Clin Exp Rheumatol. 1997 Jul-Aug;15(4):433–438. [PubMed] [Google Scholar]
- Bocchini V., Angeletti P. U. The nerve growth factor: purification as a 30,000-molecular-weight protein. Proc Natl Acad Sci U S A. 1969 Oct;64(2):787–794. doi: 10.1073/pnas.64.2.787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bürgi B., Otten U. H., Ochensberger B., Rihs S., Heese K., Ehrhard P. B., Ibanez C. F., Dahinden C. A. Basophil priming by neurotrophic factors. Activation through the trk receptor. J Immunol. 1996 Dec 15;157(12):5582–5588. [PubMed] [Google Scholar]
- Cerinic M. M., Generini S., Pignone A., Casale R. The nervous system in systemic sclerosis (scleroderma). Clinical features and pathogenetic mechanisms. Rheum Dis Clin North Am. 1996 Nov;22(4):879–892. doi: 10.1016/s0889-857x(05)70306-9. [DOI] [PubMed] [Google Scholar]
- Cerinic M. M., Konttinen Y., Generini S., Cutolo M. Neuropeptides and steroid hormones in arthritis. Curr Opin Rheumatol. 1998 May;10(3):220–235. doi: 10.1097/00002281-199805000-00011. [DOI] [PubMed] [Google Scholar]
- Di Marco E., Marchisio P. C., Bondanza S., Franzi A. T., Cancedda R., De Luca M. Growth-regulated synthesis and secretion of biologically active nerve growth factor by human keratinocytes. J Biol Chem. 1991 Nov 15;266(32):21718–21722. [PubMed] [Google Scholar]
- Dicou E., Perrot S., Menkes C. J., Masson C., Nerriere V. Nerve growth factor (NGF) autoantibodies and NGF in the synovial fluid: implications in spondylarthropathies. Autoimmunity. 1996;24(1):1–9. doi: 10.3109/08916939608995352. [DOI] [PubMed] [Google Scholar]
- Dmitrieva N., Shelton D., Rice A. S., McMahon S. B. The role of nerve growth factor in a model of visceral inflammation. Neuroscience. 1997 May;78(2):449–459. doi: 10.1016/s0306-4522(96)00575-1. [DOI] [PubMed] [Google Scholar]
- Falcini F., Cerinic M. M., Ermini M., Generini S., Lombardi A., Pignone A., Leoncini G., Tirassa P., Aloe L. Nerve growth factor circulating levels are increased in Kawasaki disease: correlation with disease activity and reduced angiotensin converting enzyme levels. J Rheumatol. 1996 Oct;23(10):1798–1802. [PubMed] [Google Scholar]
- Falcini F., Matucci Cerinic M., Lombardi A., Generini S., Pignone A., Tirassa P., Ermini M., Lepore L., Partsch G., Aloe L. Increased circulating nerve growth factor is directly correlated with disease activity in juvenile chronic arthritis. Ann Rheum Dis. 1996 Oct;55(10):745–748. doi: 10.1136/ard.55.10.745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gee A. P., Boyle M. D., Munger K. L., Lawman M. J., Young M. Nerve growth factor: stimulation of polymorphonuclear leukocyte chemotaxis in vitro. Proc Natl Acad Sci U S A. 1983 Dec;80(23):7215–7218. doi: 10.1073/pnas.80.23.7215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goss J. R., Taffe K. M., Kochanek P. M., DeKosky S. T. The antioxidant enzymes glutathione peroxidase and catalase increase following traumatic brain injury in the rat. Exp Neurol. 1997 Jul;146(1):291–294. doi: 10.1006/exnr.1997.6515. [DOI] [PubMed] [Google Scholar]
- Greene L. A., Shooter E. M. The nerve growth factor: biochemistry, synthesis, and mechanism of action. Annu Rev Neurosci. 1980;3:353–402. doi: 10.1146/annurev.ne.03.030180.002033. [DOI] [PubMed] [Google Scholar]
- Guidry G., Landis S. C., Davis B. M., Albers K. M. Overexpression of nerve growth factor in epidermis disrupts the distribution and properties of sympathetic innervation in footpads. J Comp Neurol. 1998 Apr 6;393(2):231–243. doi: 10.1002/(sici)1096-9861(19980406)393:2<231::aid-cne7>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- Kaada B. Successful treatment of esophageal dysmotility and Raynaud's phenomenon in systemic sclerosis and achalasia by transcutaneous nerve stimulation. Increase in plasma VIP concentration. Scand J Gastroenterol. 1987 Nov;22(9):1137–1146. doi: 10.3109/00365528708991971. [DOI] [PubMed] [Google Scholar]
- Kahaleh B., Matucci-Cerinic M. Raynaud's phenomenon and scleroderma. Dysregulated neuroendothelial control of vascular tone. Arthritis Rheum. 1995 Jan;38(1):1–4. doi: 10.1002/art.1780380102. [DOI] [PubMed] [Google Scholar]
- Kahaleh M. B., Sultany G. L., Smith E. A., Huffstutter J. E., Loadholt C. B., LeRoy E. C. A modified scleroderma skin scoring method. Clin Exp Rheumatol. 1986 Oct-Dec;4(4):367–369. [PubMed] [Google Scholar]
- Kannan Y., Bienenstock J., Ohta M., Stanisz A. M., Stead R. H. Nerve growth factor and cytokines mediate lymphoid tissue-induced neurite outgrowth from mouse superior cervical ganglia in vitro. J Immunol. 1996 Jul 1;157(1):313–320. [PubMed] [Google Scholar]
- Kazzam E., Caidahl K., Hedner T., Hedner J., Waldenström A. Plasma noradrenaline and neuropeptide-Y may not be of primary importance in the pathophysiology of cardiac involvement in systemic sclerosis. Scand J Rheumatol. 1999;28(4):238–243. doi: 10.1080/03009749950155616. [DOI] [PubMed] [Google Scholar]
- Laudiero L. B., Aloe L., Levi-Montalcini R., Buttinelli C., Schilter D., Gillessen S., Otten U. Multiple sclerosis patients express increased levels of beta-nerve growth factor in cerebrospinal fluid. Neurosci Lett. 1992 Nov 23;147(1):9–12. doi: 10.1016/0304-3940(92)90762-v. [DOI] [PubMed] [Google Scholar]
- LeRoy E. C., Black C., Fleischmajer R., Jablonska S., Krieg T., Medsger T. A., Jr, Rowell N., Wollheim F. Scleroderma (systemic sclerosis): classification, subsets and pathogenesis. J Rheumatol. 1988 Feb;15(2):202–205. [PubMed] [Google Scholar]
- Leon A., Buriani A., Dal Toso R., Fabris M., Romanello S., Aloe L., Levi-Montalcini R. Mast cells synthesize, store, and release nerve growth factor. Proc Natl Acad Sci U S A. 1994 Apr 26;91(9):3739–3743. doi: 10.1073/pnas.91.9.3739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levi-Montalcini R., Skaper S. D., Dal Toso R., Petrelli L., Leon A. Nerve growth factor: from neurotrophin to neurokine. Trends Neurosci. 1996 Nov;19(11):514–520. doi: 10.1016/S0166-2236(96)10058-8. [DOI] [PubMed] [Google Scholar]
- Lori S., Matucci-Cerinic M., Casale R., Generini S., Lombardi A., Pignone A., Scaletti C., Gangemi P. F., Cagnoni M. Peripheral nervous system involvement in systemic sclerosis: the median nerve as target structure. Clin Exp Rheumatol. 1996 Nov-Dec;14(6):601–605. [PubMed] [Google Scholar]
- Matucci-Cerinic M., Jaffa A., Kahaleh B. Angiotensin converting enzyme: an in vivo and in vitro marker of endothelial injury. J Lab Clin Med. 1992 Sep;120(3):428–433. [PubMed] [Google Scholar]
- Matucci-Cerinic M. Sensory neuropeptides and rheumatic diseases. Rheum Dis Clin North Am. 1993 Nov;19(4):975–991. [PubMed] [Google Scholar]
- Miller M. S., Buck S. H., Sipes I. G., Yamamura H. I., Burks T. F. Regulation of substance P by nerve growth factor: disruption by capsaicin. Brain Res. 1982 Oct 28;250(1):193–196. doi: 10.1016/0006-8993(82)90969-6. [DOI] [PubMed] [Google Scholar]
- Misra R., Darton K., Jewkes R. F., Black C. M., Maini R. N. Arthritis in scleroderma. Br J Rheumatol. 1995 Sep;34(9):831–837. doi: 10.1093/rheumatology/34.9.831. [DOI] [PubMed] [Google Scholar]
- Murase K., Murakami Y., Takayanagi K., Furukawa Y., Hayashi K. Human fibroblast cells synthesize and secrete nerve growth factor in culture. Biochem Biophys Res Commun. 1992 Apr 15;184(1):373–379. doi: 10.1016/0006-291x(92)91203-3. [DOI] [PubMed] [Google Scholar]
- Nonogaki K., Moser A. H., Shigenaga J., Feingold K. R., Grunfeld C. Beta-nerve growth factor as a mediator of the acute phase response in vivo. Biochem Biophys Res Commun. 1996 Feb 27;219(3):956–961. doi: 10.1006/bbrc.1996.0325. [DOI] [PubMed] [Google Scholar]
- Pan Z., Perez-Polo R. Increased uptake of L-cysteine and L-cystine by nerve growth factor in rat pheochromocytoma cells. Brain Res. 1996 Nov 18;740(1-2):21–26. doi: 10.1016/s0006-8993(96)00844-x. [DOI] [PubMed] [Google Scholar]
- Pan Z., Perez-Polo R. Regulation of gamma-glutamylcysteine synthetase activity by nerve growth factor. Int J Dev Neurosci. 1996 Aug;14(5):559–566. [PubMed] [Google Scholar]
- Pan Z., Perez-Polo R. Role of nerve growth factor in oxidant homeostasis: glutathione metabolism. J Neurochem. 1993 Nov;61(5):1713–1721. doi: 10.1111/j.1471-4159.1993.tb09808.x. [DOI] [PubMed] [Google Scholar]
- Safieh-Garabedian B., Poole S., Allchorne A., Winter J., Woolf C. J. Contribution of interleukin-1 beta to the inflammation-induced increase in nerve growth factor levels and inflammatory hyperalgesia. Br J Pharmacol. 1995 Aug;115(7):1265–1275. doi: 10.1111/j.1476-5381.1995.tb15035.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santambrogio L., Benedetti M., Chao M. V., Muzaffar R., Kulig K., Gabellini N., Hochwald G. Nerve growth factor production by lymphocytes. J Immunol. 1994 Nov 15;153(10):4488–4495. [PubMed] [Google Scholar]
- Santavirta N., Konttinen Y. T., Törnwall J., Segerberg M., Santavirta S., Matucci-Cerinic M., Björvell H. Neuropeptides of the autonomic nervous system in Sjögren's syndrome. Ann Rheum Dis. 1997 Dec;56(12):737–740. doi: 10.1136/ard.56.12.737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solomon A., Aloe L., Pe'er J., Frucht-Pery J., Bonini S., Bonini S., Levi-Schaffer F. Nerve growth factor is preformed in and activates human peripheral blood eosinophils. J Allergy Clin Immunol. 1998 Sep;102(3):454–460. doi: 10.1016/s0091-6749(98)70135-6. [DOI] [PubMed] [Google Scholar]
- Strehlow D., Korn J. H. Biology of the scleroderma fibroblast. Curr Opin Rheumatol. 1998 Nov;10(6):572–578. doi: 10.1097/00002281-199811000-00011. [DOI] [PubMed] [Google Scholar]
- Tirassa P., Stenfors C., Lundeberg T., Aloe L. Cholecystokinin-8 regulation of NGF concentrations in adult mouse brain through a mechanism involving CCK(A) and CCK(B) receptors. Br J Pharmacol. 1998 Mar;123(6):1230–1236. doi: 10.1038/sj.bjp.0701718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuveri M. A., Passiu G., Mathieu A., Aloe L. Nerve growth factor and mast cell distribution in the skin of patients with systemic sclerosis. Clin Exp Rheumatol. 1993 May-Jun;11(3):319–322. [PubMed] [Google Scholar]
- Weskamp G., Otten U. An enzyme-linked immunoassay for nerve growth factor (NGF): a tool for studying regulatory mechanisms involved in NGF production in brain and in peripheral tissues. J Neurochem. 1987 Jun;48(6):1779–1786. doi: 10.1111/j.1471-4159.1987.tb05736.x. [DOI] [PubMed] [Google Scholar]
- White B. Immune abnormalities in systemic sclerosis. Clin Dermatol. 1994 Jul-Sep;12(3):349–359. doi: 10.1016/0738-081x(94)90287-9. [DOI] [PubMed] [Google Scholar]
- Woolf C. J. Phenotypic modification of primary sensory neurons: the role of nerve growth factor in the production of persistent pain. Philos Trans R Soc Lond B Biol Sci. 1996 Mar 29;351(1338):441–448. doi: 10.1098/rstb.1996.0040. [DOI] [PubMed] [Google Scholar]
- Woolf C. J., Safieh-Garabedian B., Ma Q. P., Crilly P., Winter J. Nerve growth factor contributes to the generation of inflammatory sensory hypersensitivity. Neuroscience. 1994 Sep;62(2):327–331. doi: 10.1016/0306-4522(94)90366-2. [DOI] [PubMed] [Google Scholar]