Abstract
OBJECTIVES—To investigate the expression and regulation of CD80, CD86, and CD28 costimulatory molecules in sialoadenitis and interstitial nephritis in patients with Sjögren's syndrome (SS). METHODS—Expression of CD80, CD86, and CD28 molecules was studied by immunohistochemical staining of lip biopsy specimens obtained from patients who had sialoadenitis associated with SS, and renal biopsy specimens obtained from patients who had interstitial nephritis associated with SS. To elucidate the mechanism of de novo expression of CD80 and CD86 antigens, their induction by cytokines in human salivary duct cell line (HSG) and renal cortical epithelial cells (HRCE) by cell enzyme linked immunosorbent assay (ELISA) was quantitatively investigated. RESULTS—In patients with severe sialoadenitis, CD80 and CD86 were strongly expressed on ductal epithelial cells. In contrast, these antigens were not found in the minor salivary glands of normal subjects or of patients with mild sialoadenitis. Some infiltrating cells expressed CD28. In patients who had interstitial nephritis associated with SS, some tubular epithelial cells expressed CD86 but not the CD80 antigen. Unstimulated HSG cells did not express CD80 or CD86. Interferon γ (IFNγ) consistently up regulated levels of CD80 and CD86. In contrast, tumour necrosis factor α (TNFα), interleukin 1β (IL1β), IL2, and IL4 had no effect on either CD80 or CD86 levels. Unstimulated HRCE did not express CD80 or CD86. IFNγ consistently up regulated CD86 expression. No CD80 expression was found on tubular cells. TNFα, IL1β, IL2, and IL4 had no discernible effects. CONCLUSIONS—Salivary ductal cells in patients with SS can express CD80 and CD86 costimulatory molecules in response to IFNγ. Tubular epithelial cells in patients who have interstitial nephritis associated with SS express only CD86 molecules. In patients with SS, salivary ductal cells and tubular epithelial cells may activate infiltrating CD28 positive T lymphocytes by presenting antigens to T cells, potentially leading to tissue destruction.
Full Text
The Full Text of this article is available as a PDF (346.6 KB).
Figure 1 .
Immunohistochemical staining of CD80 and CD86 in the salivary gland. (A) CD80 was not seen in normal controls (×180); (B) CD86 was not seen in normal controls (×180); (C) CD80 on ductal epithelial cells (arrow) and some infiltrating mononuclear cells in severe sialoadenitis (×180); (D) CD86 was expressed on ductal epithelial cells (arrow) and some infiltrating mononuclear cells in severe sialoadenitis (×360); (E) CD28 was seen on many infiltrating mononuclear cells in severe sialoadenitis around the duct expressing CD80; (F) serial section of (E) (×180).
Figure 2 .
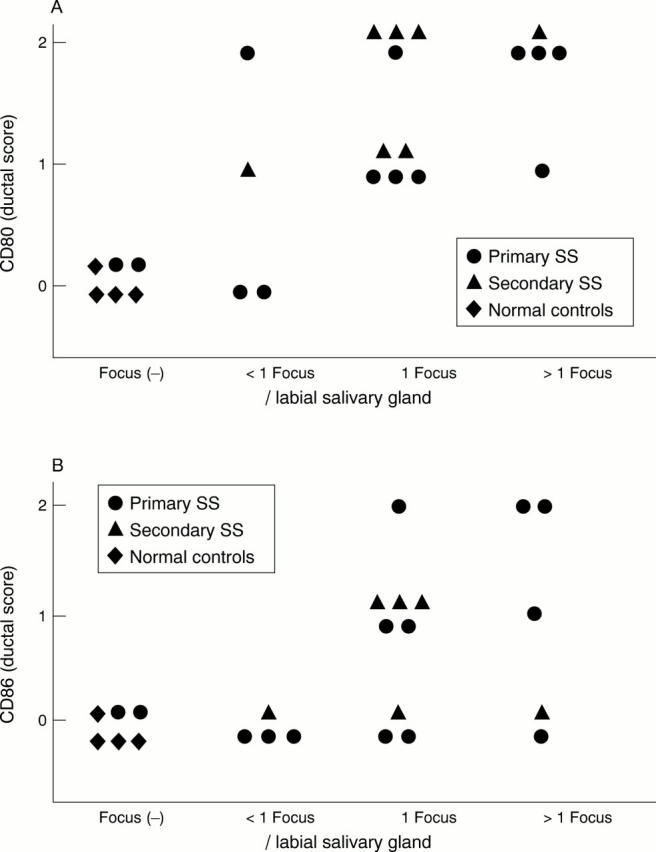
Ductal CD80 (A) and CD86 (B) expression and salivary gland lymphocytic infiltration. Ductal CD80 and CD86 expression was shown by the grading scale. CD80 and CD86 expression of ductal cells correlated with interstitial infiltration. p<0.01 in Spearman rank correlation.
Figure 3 .
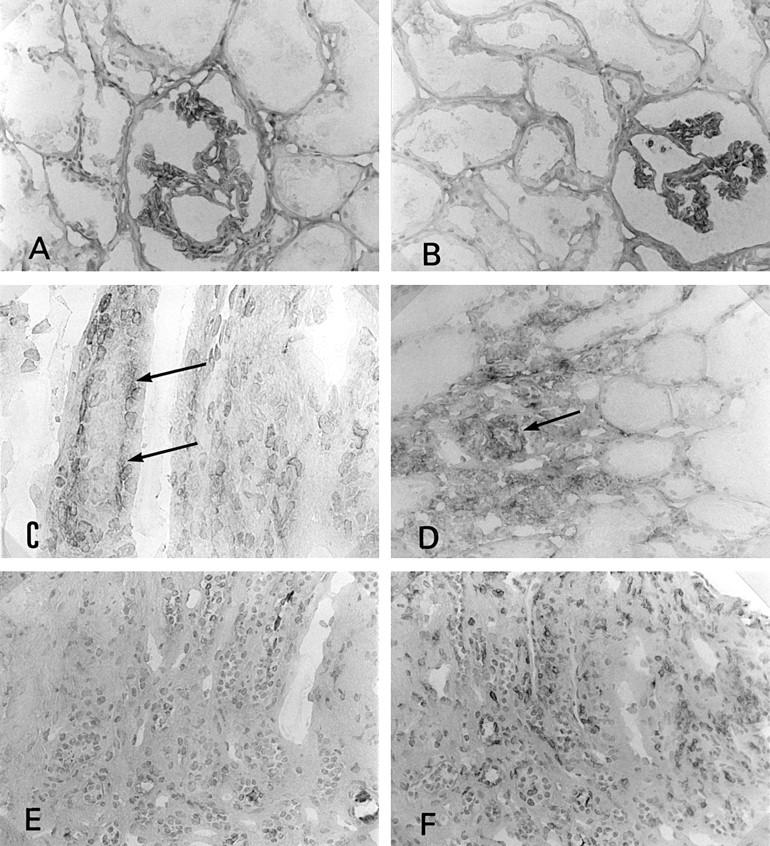
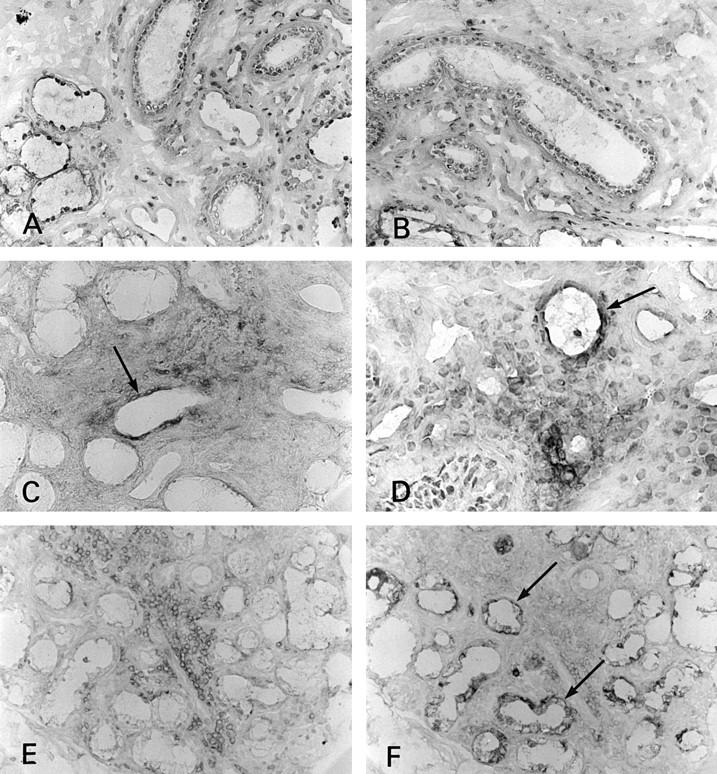
Immunostaining of CD80 and CD86 in the kidney. (A) CD80 was not seen on tubular epithelial cells in control tissue (×180); (B) CD86 was not seen on tubular epithelial cells in control tissue (×180); (C) CD86 expression on tubular epithelial cells (basal side, arrows) in interstitial nephritis associated with Sjögren's syndrome (SS) (×360); (D) the several tubular cells showed CD86 antigen in SS kidney (arrow) (×180); (E) CD80 was not seen on tubular epithelial cells in interstitial nephritis associated with SS (×180); (F) CD28 expressed on some infiltrating cells (the same portion of the serial section of (C) and (E) (×180)).
Figure 4 .
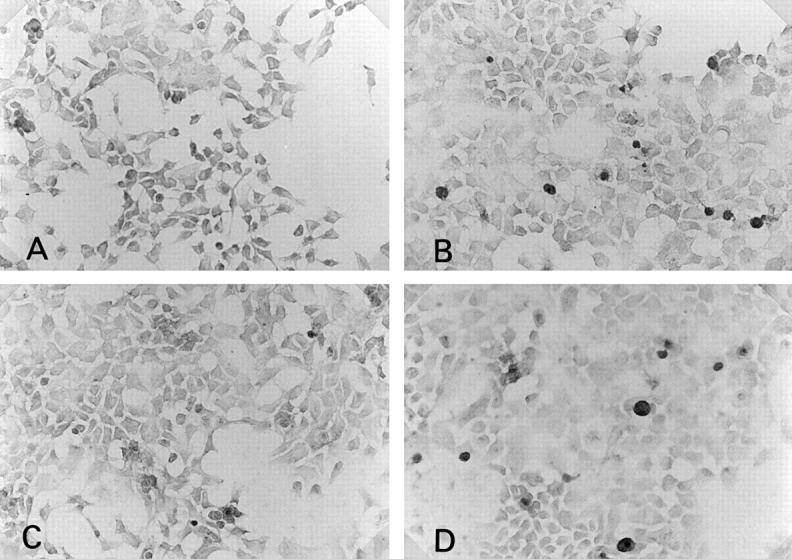
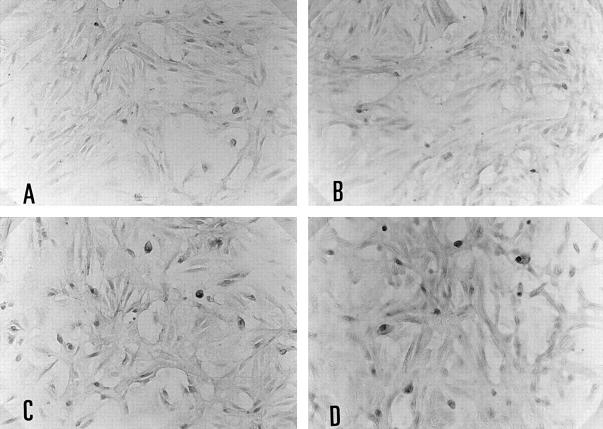
Immunohistochemical staining of anti-CD80 or CD86 antibody on cultured human salivary gland duct (HSG) cell line (×180). Without cytokine stimulation, CD80 (A) or CD86 (B) expression was not seen on HSG. Many HSG cells stimulated by 100 U/ml of interferon γ for 24 hours showed CD80 (C) or CD86 (D) expression.
Figure 5 .
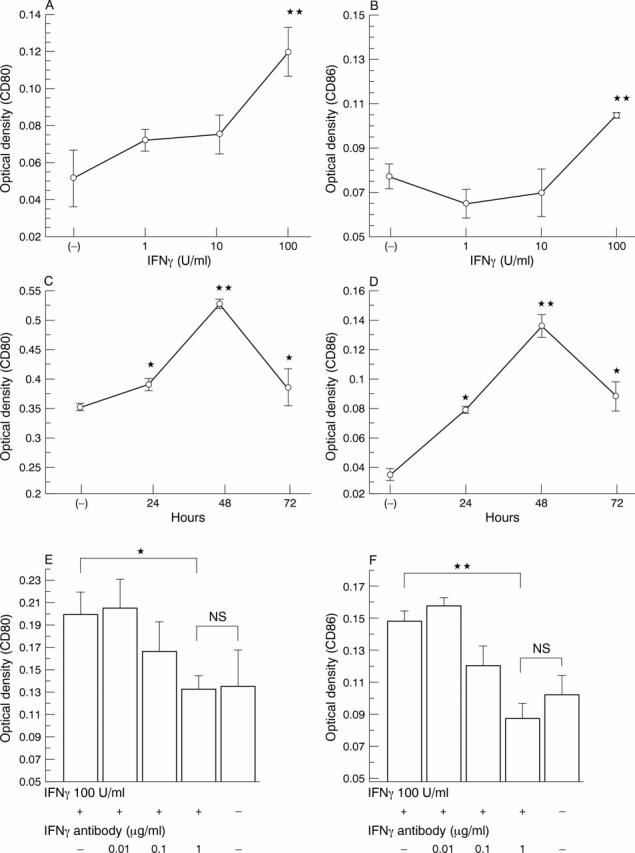
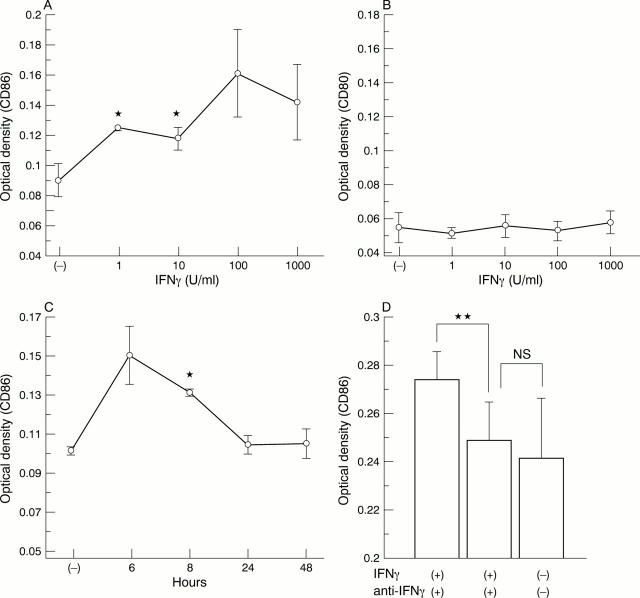
Effect of interferon γ (IFNγ) on human salivary gland duct (HSG) CD80 or CD86 expression as measured by immunoperoxidase cell ELISA. Cells were cultured and stimulated with IFNγ. (A) CD80 expression after 24 hours' exposure to increasing concentrations of IFNγ; (B) CD86 expression after 24 hours' exposure to increasing concentrations of IFNγ; (C) time course of induction of CD80 by IFNγ (100 U/ml); (D) time course of induction of CD86 by IFNγ (100 U/ml); (E, F) effect of blockade of CD80 (E) and CD86 (F) expression by anti-IFNγ. HSG cells were cultured and stimulated with 100 U/ml of IFNγ and with 0.01, 0.1, and 1 µg/ml of monoclonal anti-IFNγ antibody (R&D). Addition of 1 µg/ml of anti-IFNγ inhibited the up regulation of IFNγ. All values are expressed as means (SD) of triplicate wells. *p<0.05, **p<0.01 indicate significant difference from the control (untreated) OD. Absence of an error bar indicates that the error was less than the symbol size.
Figure 6 .
Immunohistochemical staining of anti-CD80 or CD86 antibody on cultured renal cortical epithelial cells (HRCE) cells (×180). Without cytokine stimulation, CD80 (A) expression was not seen on HRCE and a few CD86 positive cells were seen (C). CD80 expression was not induced by interferon γ (IFNγ) (B). Many HRCE cells stimulated by 100 U/ml IFNγ for 24 hours showed only CD86 (D) expression.
Figure 7 .
Effect of interferon γ (IFNγ) in CD80 or CD86 expression on renal cortical epithelial (HRCE) cells as measured by immunoperoxidase cell ELISA. Cells were cultured and stimulated with IFNγ. (A) CD86 expression after 12 hours' exposure to increasing concentrations of IFNγ; (B) CD80 expression was not induced by 12 hours' exposure to IFNγ; (C) Time course of induction of CD86 by IFNγ (100 U/ml); (D) effect of blockade of CD86 expression by anti-IFNγ (a-IFN). HRCE cells were cultured and stimulated with 100 U/ml of IFNγ and 1 µg/ml polyclonal anti-IFNγ (Genzyme). Addition of 1 µg/ml of anti-IFNγ inhibited the up regulation of IFNγ. All values are expressed as means (SD) of triplicate wells. *p<0.05, **p<0.01 indicate a significant difference from control (untreated) OD. No error bar indicates that the error was less than the symbol size.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adamson T. C., 3rd, Fox R. I., Frisman D. M., Howell F. V. Immunohistologic analysis of lymphoid infiltrates in primary Sjogren's syndrome using monoclonal antibodies. J Immunol. 1983 Jan;130(1):203–208. [PubMed] [Google Scholar]
- Azuma M., Ito D., Yagita H., Okumura K., Phillips J. H., Lanier L. L., Somoza C. B70 antigen is a second ligand for CTLA-4 and CD28. Nature. 1993 Nov 4;366(6450):76–79. doi: 10.1038/366076a0. [DOI] [PubMed] [Google Scholar]
- Balsa A., Dixey J., Sansom D. M., Maddison P. J., Hall N. D. Differential expression of the costimulatory molecules B7.1 (CD80) and B7.2 (CD86) in rheumatoid synovial tissue. Br J Rheumatol. 1996 Jan;35(1):33–37. doi: 10.1093/rheumatology/35.1.33. [DOI] [PubMed] [Google Scholar]
- Banu N., Meyers C. M. IFN-gamma and LPS differentially modulate class II MHC and B7-1 expression on murine renal tubular epithelial cells. Kidney Int. 1999 Jun;55(6):2250–2263. doi: 10.1046/j.1523-1755.1999.00495.x. [DOI] [PubMed] [Google Scholar]
- Benson E. M., Colvin R. B., Russell P. S. Induction of IA antigens in murine renal transplants. J Immunol. 1985 Jan;134(1):7–9. [PubMed] [Google Scholar]
- Boumba D., Skopouli F. N., Moutsopoulos H. M. Cytokine mRNA expression in the labial salivary gland tissues from patients with primary Sjögren's syndrome. Br J Rheumatol. 1995 Apr;34(4):326–333. doi: 10.1093/rheumatology/34.4.326. [DOI] [PubMed] [Google Scholar]
- Denfeld R. W., Kind P., Sontheimer R. D., Schöpf E., Simon J. C. In situ expression of B7 and CD28 receptor families in skin lesions of patients with lupus erythematosus. Arthritis Rheum. 1997 May;40(5):814–821. doi: 10.1002/art.1780400507. [DOI] [PubMed] [Google Scholar]
- Fox R. I., Kang H. I., Ando D., Abrams J., Pisa E. Cytokine mRNA expression in salivary gland biopsies of Sjögren's syndrome. J Immunol. 1994 Jun 1;152(11):5532–5539. [PubMed] [Google Scholar]
- Freeman G. J., Boussiotis V. A., Anumanthan A., Bernstein G. M., Ke X. Y., Rennert P. D., Gray G. S., Gribben J. G., Nadler L. M. B7-1 and B7-2 do not deliver identical costimulatory signals, since B7-2 but not B7-1 preferentially costimulates the initial production of IL-4. Immunity. 1995 May;2(5):523–532. doi: 10.1016/1074-7613(95)90032-2. [DOI] [PubMed] [Google Scholar]
- Gerhardt R. E., Loebl D. H., Rao R. N. Interstitial immunofluorescence in nephritis of Sjögren's syndrome. Clin Nephrol. 1978 Nov;10(5):201–207. [PubMed] [Google Scholar]
- Grunow R., D'Apuzzo M., Wyss-Coray T., Frutig K., Pichler W. J. A cell surface ELISA for the screening of monoclonal antibodies to antigens on viable cells in suspension. J Immunol Methods. 1994 May 2;171(1):93–102. doi: 10.1016/0022-1759(94)90232-1. [DOI] [PubMed] [Google Scholar]
- Hagerty D. T. Intercellular adhesion molecule-1 is necessary but not sufficient to activate CD4+ T cells. Discovery of a novel costimulator on kidney tubule cells. J Immunol. 1996 May 15;156(10):3652–3659. [PubMed] [Google Scholar]
- Harding F. A., McArthur J. G., Gross J. A., Raulet D. H., Allison J. P. CD28-mediated signalling co-stimulates murine T cells and prevents induction of anergy in T-cell clones. Nature. 1992 Apr 16;356(6370):607–609. doi: 10.1038/356607a0. [DOI] [PubMed] [Google Scholar]
- Katz J., Nagler R., Barak S., Livneh A., Baum B., Atkinson J., Shemer J. Cytokines modulate interleukin-6 production by human salivary gland cell line. Cell Immunol. 1994 Dec;159(2):211–219. doi: 10.1006/cimm.1994.1308. [DOI] [PubMed] [Google Scholar]
- Kuchroo V. K., Das M. P., Brown J. A., Ranger A. M., Zamvil S. S., Sobel R. A., Weiner H. L., Nabavi N., Glimcher L. H. B7-1 and B7-2 costimulatory molecules activate differentially the Th1/Th2 developmental pathways: application to autoimmune disease therapy. Cell. 1995 Mar 10;80(5):707–718. doi: 10.1016/0092-8674(95)90349-6. [DOI] [PubMed] [Google Scholar]
- Levine B. L., Ueda Y., Craighead N., Huang M. L., June C. H. CD28 ligands CD80 (B7-1) and CD86 (B7-2) induce long-term autocrine growth of CD4+ T cells and induce similar patterns of cytokine secretion in vitro. Int Immunol. 1995 Jun;7(6):891–904. doi: 10.1093/intimm/7.6.891. [DOI] [PubMed] [Google Scholar]
- Liu M. F., Kohsaka H., Sakurai H., Azuma M., Okumura K., Saito I., Miyasaka N. The presence of costimulatory molecules CD86 and CD28 in rheumatoid arthritis synovium. Arthritis Rheum. 1996 Jan;39(1):110–114. doi: 10.1002/art.1780390115. [DOI] [PubMed] [Google Scholar]
- Manoussakis M. N., Dimitriou I. D., Kapsogeorgou E. K., Xanthou G., Paikos S., Polihronis M., Moutsopoulos H. M. Expression of B7 costimulatory molecules by salivary gland epithelial cells in patients with Sjögren's syndrome. Arthritis Rheum. 1999 Feb;42(2):229–239. doi: 10.1002/1529-0131(199902)42:2<229::AID-ANR4>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- Matsumura R., Kagami M., Tomioka H., Tanabe E., Sugiyama T., Sueishi M., Nakajima A., Azuma M., Okumura K. Expression of ductal Fas antigen in sialoadenitis of Sjögren's syndrome. Clin Exp Rheumatol. 1996 May-Jun;14(3):309–311. [PubMed] [Google Scholar]
- Matsumura R., Kondo Y., Sugiyama T., Sueishi M., Koike T., Takabayashi K., Tomioka H., Yoshida S., Tsuchida H. Immunohistochemical identification of infiltrating mononuclear cells in tubulointerstitial nephritis associated with Sjögren's syndrome. Clin Nephrol. 1988 Dec;30(6):335–340. [PubMed] [Google Scholar]
- Matsumura R., Umemiya K., Kagami M., Tomioka H., Tanabe E., Sugiyama T., Sueishi M., Nakajima A., Azuma M., Okumura K. Glandular and extraglandular expression of the Fas-Fas ligand and apoptosis in patients with Sjögren's syndrome. Clin Exp Rheumatol. 1998 Sep-Oct;16(5):561–568. [PubMed] [Google Scholar]
- Matsumura R., Umemiya K., Nakazawa T., Ochiai K., Kagami M., Tomioka H., Tanabe E., Sugiyama T., Sueishi M. Expression of cell adhesion molecules in tubulointerstitial nephritis associated with Sjögren's syndrome. Clin Nephrol. 1998 Feb;49(2):74–81. [PubMed] [Google Scholar]
- Moutsopoulos H. M., Hooks J. J., Chan C. C., Dalavanga Y. A., Skopouli F. N., Detrick B. HLA-DR expression by labial minor salivary gland tissues in Sjögren's syndrome. Ann Rheum Dis. 1986 Aug;45(8):677–683. doi: 10.1136/ard.45.8.677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller D. L., Jenkins M. K., Schwartz R. H. Clonal expansion versus functional clonal inactivation: a costimulatory signalling pathway determines the outcome of T cell antigen receptor occupancy. Annu Rev Immunol. 1989;7:445–480. doi: 10.1146/annurev.iy.07.040189.002305. [DOI] [PubMed] [Google Scholar]
- Murata H., Kita Y., Sakamoto A., Matsumoto I., Matsumura R., Sugiyama T., Sueishi M., Takabayashi K., Iwamoto I., Saitoh Y. Limited TCR repertoire of infiltrating T cells in the kidneys of Sjögren's syndrome patients with interstitial nephritis. J Immunol. 1995 Oct 15;155(8):4084–4089. [PubMed] [Google Scholar]
- Oxholm P., Daniels T. E., Bendtzen K. Cytokine expression in labial salivary glands from patients with primary Sjögren's syndrome. Autoimmunity. 1992;12(3):185–191. doi: 10.3109/08916939209148458. [DOI] [PubMed] [Google Scholar]
- Polihronis M., Tapinos N. I., Theocharis S. E., Economou A., Kittas C., Moutsopoulos H. M. Modes of epithelial cell death and repair in Sjögren's syndrome (SS). Clin Exp Immunol. 1998 Dec;114(3):485–490. doi: 10.1046/j.1365-2249.1998.00705.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulz H., Karau A., Filsinger S., Schoels M., Kabelitz D., Richter R., Hänsch G. M. Tubular epithelial cells as accessory cells for superantigen-induced T cell activation. Exp Nephrol. 1998 Jan-Feb;6(1):67–73. doi: 10.1159/000020506. [DOI] [PubMed] [Google Scholar]
- Schwartz R. H. A cell culture model for T lymphocyte clonal anergy. Science. 1990 Jun 15;248(4961):1349–1356. doi: 10.1126/science.2113314. [DOI] [PubMed] [Google Scholar]
- Seino K., Azuma M., Bashuda H., Fukao K., Yagita H., Okumura K. CD86 (B70/B7-2) on endothelial cells co-stimulates allogeneic CD4+ T cells. Int Immunol. 1995 Aug;7(8):1331–1337. doi: 10.1093/intimm/7.8.1331. [DOI] [PubMed] [Google Scholar]
- Sharp G. C., Irvin W. S., Tan E. M., Gould R. G., Holman H. R. Mixed connective tissue disease--an apparently distinct rheumatic disease syndrome associated with a specific antibody to an extractable nuclear antigen (ENA). Am J Med. 1972 Feb;52(2):148–159. doi: 10.1016/0002-9343(72)90064-2. [DOI] [PubMed] [Google Scholar]
- Shirasuna K., Sato M., Miyazaki T. A neoplastic epithelial duct cell line established from an irradiated human salivary gland. Cancer. 1981 Aug 1;48(3):745–752. doi: 10.1002/1097-0142(19810801)48:3<745::aid-cncr2820480314>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- Sumida T., Yonaha F., Maeda T., Tanabe E., Koike T., Tomioka H., Yoshida S. T cell receptor repertoire of infiltrating T cells in lips of Sjögren's syndrome patients. J Clin Invest. 1992 Feb;89(2):681–685. doi: 10.1172/JCI115635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takasaki Y., Ogaki M., Abe K., Takeuchi K., Ando S., Tokano Y., Kobayashi S., Sekigawa I., Tsuda H., Hashimoto H. Expression of costimulatory molecule CD80 on peripheral blood T cells in patients with systemic lupus erythematosus. J Rheumatol. 1998 Jun;25(6):1085–1091. [PubMed] [Google Scholar]
- Thompson C. B., Lindsten T., Ledbetter J. A., Kunkel S. L., Young H. A., Emerson S. G., Leiden J. M., June C. H. CD28 activation pathway regulates the production of multiple T-cell-derived lymphokines/cytokines. Proc Natl Acad Sci U S A. 1989 Feb;86(4):1333–1337. doi: 10.1073/pnas.86.4.1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trifillis A. L., Regec A. L., Trump B. F. Isolation, culture and characterization of human renal tubular cells. J Urol. 1985 Feb;133(2):324–329. doi: 10.1016/s0022-5347(17)48932-4. [DOI] [PubMed] [Google Scholar]
- Winiski A. P., Foster C. A. ICAM-1 expression in a spontaneously transformed human keratinocyte cell line: characterization by a simple cell-ELISA assay. J Invest Dermatol. 1992 Jul;99(1):48–52. doi: 10.1111/1523-1747.ep12611715. [DOI] [PubMed] [Google Scholar]
- Wu A. J., Kurrasch R. H., Katz J., Fox P. C., Baum B. J., Atkinson J. C. Effect of tumor necrosis factor-alpha and interferon-gamma on the growth of a human salivary gland cell line. J Cell Physiol. 1994 Nov;161(2):217–226. doi: 10.1002/jcp.1041610205. [DOI] [PubMed] [Google Scholar]