Abstract
BACKGROUND—Systemic sclerosis (SSc, scleroderma) in either its diffuse or limited skin forms has a high mortality when vital organs are affected. No treatment has been shown to influence the outcome or significantly affect the skin score, though many forms of immunosuppression have been tried. Recent developments in haemopoietic stem cell transplantation (HSCT) have allowed the application of profound immunosuppression followed by HSCT, or rescue, to autoimmune diseases such as SSc. METHODS—Results for 41 patients included in continuing multicentre open phase I/II studies using HSCT in the treatment of poor prognosis SSc are reported. Thirty seven patients had a predominantly diffuse skin form of the disease and four the limited form, with some clinical overlap. Median age was 41 years with a 5:1 female to male ratio. The skin score was >50% of maximum in 20/33 (61%) patients, with some lung disease attributable to SSc in 28/37 (76%), the forced vital capacity being <70% of the predicted value in 18/36 (50%). Pulmonary hypertension was described in 7/37 (19%) patients and renal disease in 5/37 (14%). The Scl-70 antibody was positive in 18/32 (56%) and the anticentromere antibody in 10% of evaluable patients. Peripheral blood stem cell mobilisation was performed with cyclophosphamide or granulocyte colony stimulating factor, alone or in combination. Thirty eight patients had ex vivo CD34 stem cell selection, with additional T cell depletion in seven. Seven conditioning regimens were used, but six of these used haemoimmunoablative doses of cyclophosphamide +/- anti-thymocyte globulin +/- total body irradiation. The median duration of follow up was 12 months (3-55). RESULTS—An improvement in skin score of >25% after transplantation occurred in 20/29 (69%) evaluable patients, and deterioration in 2/29 (7%). Lung function did not change significantly after transplantation. One of five renal cases deteriorated but with no new occurrences of renal disease after HSCT, and the pulmonary hypertension did not progress in the evaluable cases. Disease progression was seen in 7/37 (19%) patients after HSCT with a median period of 67 (range 49-255) days. Eleven (27%) patients had died at census and seven (17%) deaths were considered to be related to the procedure (direct organ toxicity in four, haemorrhage in two, and infection/neutropenic fever in one). The cumulative probability of survival at one year was 73% (95% CI 58 to 88) by Kaplan-Meier analysis. CONCLUSION—Despite a higher procedure related mortality rate from HSCT in SSc compared with patients with breast cancer and non-Hodgkin's lymphoma, the marked impact on skin score, a surrogate marker of mortality, the trend towards stabilisation of lung involvement, and lack of other treatment alternatives justify further carefully designed studies. If future trials incorporate inclusion and exclusion criteria based on this preliminary experience, the predicted procedure related mortality should be around 10%.
Full Text
The Full Text of this article is available as a PDF (199.4 KB).
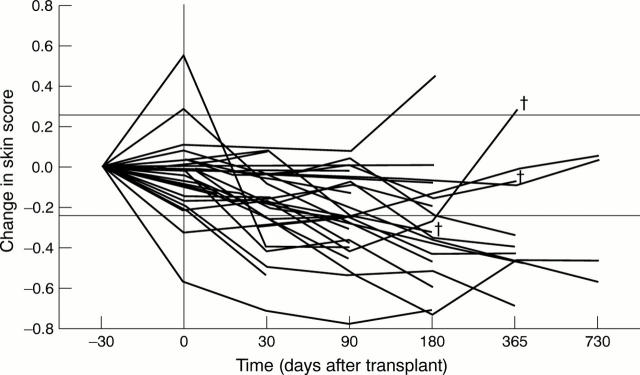
Figure 1 .
Change in skin score. The serial skin score data are presented for the 37 patients with diffuse scleroderma. The proportional change from baseline measurement was calculated for each patient at each available time point. Two horizontal lines are marked, which represent changes of 25%. The x axis is not drawn to scale. Data obtained before administration of the priming regimen are shown at -30 days (although the temporal relation to conditioning was variable). A vertical line is drawn to show the timing of conditioning treatment. † = patient death, but is only shown for deaths beyond 90 days.
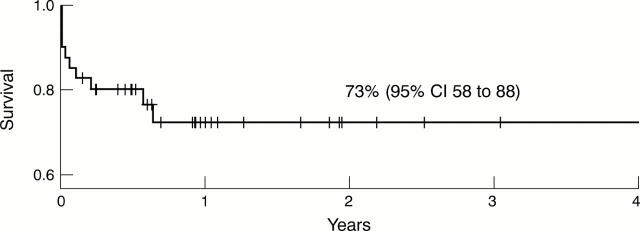
Figure 2 .
Cumulative probability of survival. A Kaplan-Meier plot of cumulative survival is shown. Survival data at census were analysed using SPSS software.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Black C. M., Silman A. J., Herrick A. I., Denton C. P., Wilson H., Newman J., Pompon L., Shi-Wen X. Interferon-alpha does not improve outcome at one year in patients with diffuse cutaneous scleroderma: results of a randomized, double-blind, placebo-controlled trial. Arthritis Rheum. 1999 Feb;42(2):299–305. doi: 10.1002/1529-0131(199902)42:2<299::AID-ANR12>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- Blockmans D., Beyens G., Verhaeghe R. Predictive value of nailfold capillaroscopy in the diagnosis of connective tissue diseases. Clin Rheumatol. 1996 Mar;15(2):148–153. doi: 10.1007/BF02230332. [DOI] [PubMed] [Google Scholar]
- Boyd R. L., Wilson T. J., Van De Water J., Haapanen L. A., Gershwin M. E. Selective abnormalities in the thymic microenvironment associated with avian scleroderma, an inherited fibrotic disease of L200 chickens. J Autoimmun. 1991 Apr;4(2):369–380. doi: 10.1016/0896-8411(91)90031-7. [DOI] [PubMed] [Google Scholar]
- Bryan C., Knight C., Black C. M., Silman A. J. Prediction of five-year survival following presentation with scleroderma: development of a simple model using three disease factors at first visit. Arthritis Rheum. 1999 Dec;42(12):2660–2665. doi: 10.1002/1529-0131(199912)42:12<2660::AID-ANR23>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- Clements P. J., Furst D. E., Wong W. K., Mayes M., White B., Wigley F., Weisman M. H., Barr W., Moreland L. W., Medsger T. A., Jr High-dose versus low-dose D-penicillamine in early diffuse systemic sclerosis: analysis of a two-year, double-blind, randomized, controlled clinical trial. Arthritis Rheum. 1999 Jun;42(6):1194–1203. doi: 10.1002/1529-0131(199906)42:6<1194::AID-ANR16>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- Clements P. J., Hurwitz E. L., Wong W. K., Seibold J. R., Mayes M., White B., Wigley F., Weisman M., Barr W., Moreland L. Skin thickness score as a predictor and correlate of outcome in systemic sclerosis: high-dose versus low-dose penicillamine trial. Arthritis Rheum. 2000 Nov;43(11):2445–2454. doi: 10.1002/1529-0131(200011)43:11<2445::AID-ANR11>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- Evans P. C., Lambert N., Maloney S., Furst D. E., Moore J. M., Nelson J. L. Long-term fetal microchimerism in peripheral blood mononuclear cell subsets in healthy women and women with scleroderma. Blood. 1999 Mar 15;93(6):2033–2037. [PubMed] [Google Scholar]
- Fanning G. C., Welsh K. I., Bunn C., Du Bois R., Black C. M. HLA associations in three mutually exclusive autoantibody subgroups in UK systemic sclerosis patients. Br J Rheumatol. 1998 Feb;37(2):201–207. doi: 10.1093/rheumatology/37.2.201. [DOI] [PubMed] [Google Scholar]
- Furst D. E. The endothelium in the pathogenesis of systemic sclerosis: is it primary or secondary? J Mal Vasc. 1999 May;24(2):95–98. [PubMed] [Google Scholar]
- Harrison N. K., Myers A. R., Corrin B., Soosay G., Dewar A., Black C. M., Du Bois R. M., Turner-Warwick M. Structural features of interstitial lung disease in systemic sclerosis. Am Rev Respir Dis. 1991 Sep;144(3 Pt 1):706–713. doi: 10.1164/ajrccm/144.3_Pt_1.706. [DOI] [PubMed] [Google Scholar]
- Haslam P. L. Evaluation of alveolitis by studies of lung biopsies. Lung. 1990;168 (Suppl):984–992. doi: 10.1007/BF02718236. [DOI] [PubMed] [Google Scholar]
- Janin-Mercier A., Saurat J. H., Bourges M., Sohier J., Jean L. D., Gluckman E. The lichen planus like and sclerotic phases of the graft versus host disease in man: an ultrastructural study of six cases. Acta Derm Venereol. 1981;61(3):187–193. [PubMed] [Google Scholar]
- Klings E. S., Hill N. S., Ieong M. H., Simms R. W., Korn J. H., Farber H. W. Systemic sclerosis-associated pulmonary hypertension: short- and long-term effects of epoprostenol (prostacyclin). Arthritis Rheum. 1999 Dec;42(12):2638–2645. doi: 10.1002/1529-0131(199912)42:12<2638::AID-ANR20>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- Martini A., Maccario R., Ravelli A., Montagna D., De Benedetti F., Bonetti F., Viola S., Zecca M., Perotti C., Locatelli F. Marked and sustained improvement two years after autologous stem cell transplantation in a girl with systemic sclerosis. Arthritis Rheum. 1999 Apr;42(4):807–811. doi: 10.1002/1529-0131(199904)42:4<807::AID-ANR26>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- Nelson J. L., Furst D. E., Maloney S., Gooley T., Evans P. C., Smith A., Bean M. A., Ober C., Bianchi D. W. Microchimerism and HLA-compatible relationships of pregnancy in scleroderma. Lancet. 1998 Feb 21;351(9102):559–562. doi: 10.1016/S0140-6736(97)08357-8. [DOI] [PubMed] [Google Scholar]
- Okunieff P., Barrett A. J., Phang S. E., Li A., Constine L. S., Williams J. P., Rubin P., Wang X., Wu T., Chen Y. Circulating basic fibroblast growth factor declines during Cy/TBI bone marrow transplantation. Bone Marrow Transplant. 1999 Jun;23(11):1117–1121. doi: 10.1038/sj.bmt.1701778. [DOI] [PubMed] [Google Scholar]
- Roumm A. D., Whiteside T. L., Medsger T. A., Jr, Rodnan G. P. Lymphocytes in the skin of patients with progressive systemic sclerosis. Quantification, subtyping, and clinical correlations. Arthritis Rheum. 1984 Jun;27(6):645–653. doi: 10.1002/art.1780270607. [DOI] [PubMed] [Google Scholar]
- Shulman H. M., Sullivan K. M., Weiden P. L., McDonald G. B., Striker G. E., Sale G. E., Hackman R., Tsoi M. S., Storb R., Thomas E. D. Chronic graft-versus-host syndrome in man. A long-term clinicopathologic study of 20 Seattle patients. Am J Med. 1980 Aug;69(2):204–217. doi: 10.1016/0002-9343(80)90380-0. [DOI] [PubMed] [Google Scholar]
- Snowden J. A., Brooks P. M., Biggs J. C. Haemopoietic stem cell transplantation for autoimmune diseases. Br J Haematol. 1997 Oct;99(1):9–22. doi: 10.1046/j.1365-2141.1997.3273144.x. [DOI] [PubMed] [Google Scholar]
- Tyndall A., Fassas A., Passweg J., Ruiz de Elvira C., Attal M., Brooks P., Black C., Durez P., Finke J., Forman S. Autologous haematopoietic stem cell transplants for autoimmune disease--feasibility and transplant-related mortality. Autoimmune Disease and Lymphoma Working Parties of the European Group for Blood and Marrow Transplantation, the European League Against Rheumatism and the International Stem Cell Project for Autoimmune Disease. Bone Marrow Transplant. 1999 Oct;24(7):729–734. doi: 10.1038/sj.bmt.1701987. [DOI] [PubMed] [Google Scholar]
- Tyndall A., Gratwohl A. Blood and marrow stem cell transplants in autoimmune disease. A consensus report written on behalf of the European League Against Rheumatism (EULAR) and the European Group for Blood and Marrow Transplantation (EBMT). Br J Rheumatol. 1997 Mar;36(3):390–392. doi: 10.1093/rheumatology/36.3.390. [DOI] [PubMed] [Google Scholar]
- Tyndall A., Gratwohl A. Hemopoietic blood and marrow transplants in the treatment of severe autoimmune disease. Curr Opin Hematol. 1997 Nov;4(6):390–394. doi: 10.1097/00062752-199704060-00005. [DOI] [PubMed] [Google Scholar]
- Weiner E. S., Hildebrandt S., Senécal J. L., Daniels L., Noell S., Joyal F., Roussin A., Earnshaw W., Rothfield N. F. Prognostic significance of anticentromere antibodies and anti-topoisomerase I antibodies in Raynaud's disease. A prospective study. Arthritis Rheum. 1991 Jan;34(1):68–77. doi: 10.1002/art.1780340111. [DOI] [PubMed] [Google Scholar]
- Wells A. U., Lorimer S., Majumdar S., Harrison N. K., Corrin B., Black C. M., Jeffery P. K., du Bois R. M. Fibrosing alveolitis in systemic sclerosis: increase in memory T-cells in lung interstitium. Eur Respir J. 1995 Feb;8(2):266–271. doi: 10.1183/09031936.95.08020266. [DOI] [PubMed] [Google Scholar]
- White B. Immunopathogenesis of systemic sclerosis. Rheum Dis Clin North Am. 1996 Nov;22(4):695–708. doi: 10.1016/s0889-857x(05)70296-9. [DOI] [PubMed] [Google Scholar]
- Wilson T. J., Van de Water J., Mohr F. C., Boyd R. L., Ansari A., Wick G., Gershwin M. E. Avian scleroderma: evidence for qualitative and quantitative T cell defects. J Autoimmun. 1992 Jun;5(3):261–276. doi: 10.1016/0896-8411(92)90142-d. [DOI] [PubMed] [Google Scholar]
- Yurovsky V. V., Sutton P. A., Schulze D. H., Wigley F. M., Wise R. A., Howard R. F., White B. Expansion of selected V delta 1+ gamma delta T cells in systemic sclerosis patients. J Immunol. 1994 Jul 15;153(2):881–891. [PubMed] [Google Scholar]
- Yurovsky V. V., Wigley F. M., Wise R. A., White B. Skewing of the CD8+ T-cell repertoire in the lungs of patients with systemic sclerosis. Hum Immunol. 1996 Jun-Jul;48(1-2):84–97. doi: 10.1016/0198-8859(96)00091-2. [DOI] [PubMed] [Google Scholar]
- van de Water J., Haapanen L., Boyd R., Abplanalp H., Gershwin M. E. Identification of T cells in early dermal lymphocytic infiltrates in avian scleroderma. Arthritis Rheum. 1989 Aug;32(8):1031–1040. doi: 10.1002/anr.1780320813. [DOI] [PubMed] [Google Scholar]
- van den Hoogen F. H., Boerbooms A. M., Swaak A. J., Rasker J. J., van Lier H. J., van de Putte L. B. Comparison of methotrexate with placebo in the treatment of systemic sclerosis: a 24 week randomized double-blind trial, followed by a 24 week observational trial. Br J Rheumatol. 1996 Apr;35(4):364–372. doi: 10.1093/rheumatology/35.4.364. [DOI] [PubMed] [Google Scholar]