Abstract
OBJECTIVE—To evaluate oxidative injury and inflammatory status in various rheumatic diseases by measuring the levels of isoprostanes and prostaglandins in serum and synovial fluid. METHODS—The concentrations of 8-iso-PGF2α (F2-isoprostane indicating oxidative injury) and 15-keto-dihydro-PGF2α (a major metabolite of prostaglandin F2α) were measured in both serum and synovial fluid aspirated from 26 patients with various arthritic diseases, including rheumatoid arthritis (RA), reactive arthritis (ReA), psoriatic arthritis (PsA), and osteoarthritis (OA). These prostaglandin derivatives were also measured in serum samples collected from 42 healthy control subjects. RESULTS—Overall, serum levels of 8-iso-PGF2α and 15-keto-dihydro-PGF2α were much higher in patients with arthritic diseases than in the healthy control subjects. The levels of 8-iso-PGF2α and 15-keto-dihydro-PGF2α in synovial fluid aspirated from knee joints were also high and varied among various types of arthritic patients. Although the synovial fluid level of these prostaglandin derivatives was sometimes higher than in the corresponding serum sample, this was not a consistent finding. Overall, there was no correlation between serum and synovial fluid levels of 8-iso-PGF2α, or between serum and synovial fluid levels of 15-keto-dihydro-PGF2α. However, a strong relation was found between the levels of 8-iso-PGF2α and 15-keto-dihydro-PGF2α, in both serum (rs=0.53, p<0.001) and synovial fluid (rs=0.62, p<0.001). CONCLUSIONS—These data suggest that both free radical mediated oxidative injury and cyclo-oxygenase dependent inflammatory responses are closely correlated in various types of arthritis.
Full Text
The Full Text of this article is available as a PDF (153.7 KB).
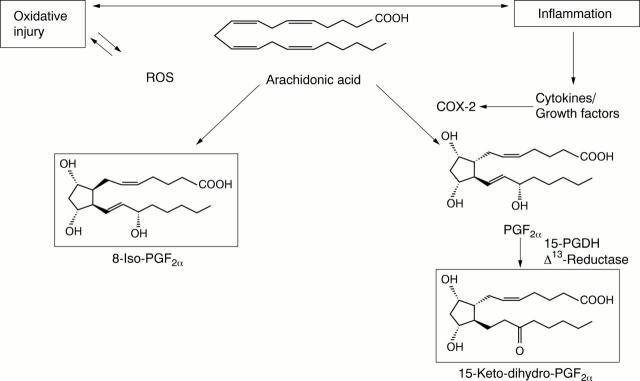
Figure 1 .
Schematic diagram of relation between inflammation and oxidative injury, and endogenous formation of 8-iso-PGF2α through free radical and 15-keto-dihydro-PGF2α via cyclo-oxygenase catalysed oxidation of arachidonic acid. ROS = reactive oxygen species; COX = cyclo-oxygenase; 15-PGDH = 15-hydroxyprostaglandin dehydrogenase, Δ13-reductase.
Figure 2 .
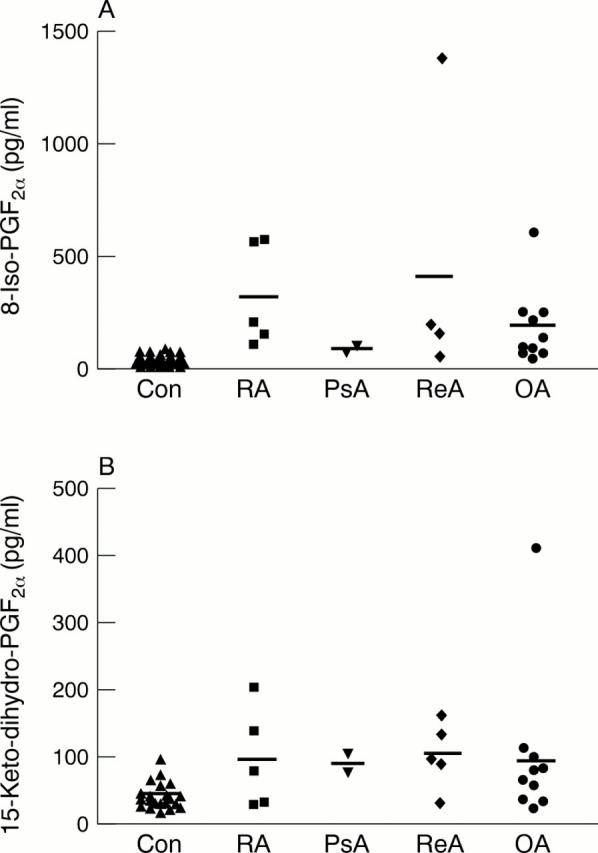
Serum levels of (A) 8-iso-PGF2α and (B) 15-keto-dihydro-PGF2α in individual patients with various types of rheumatic disease, and controls. The mean level is represented by a bar in each group.
Figure 3 .
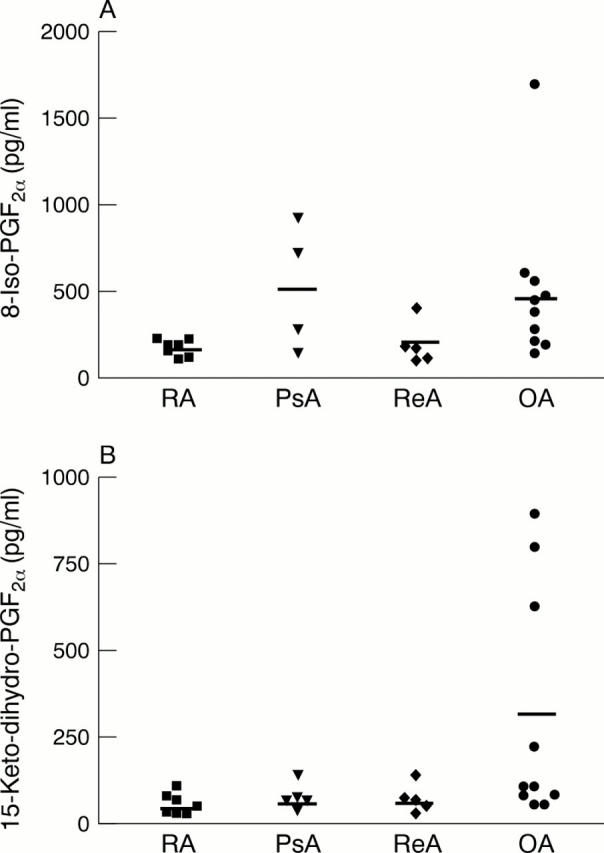
Synovial fluid levels of (A) 8-iso-PGF2α and (B) 15-keto-dihydro-PGF2α in individual patients with various types of rheumatic disease, and controls. The mean level is represented by a bar in each group.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ames P. R., Alves J., Murat I., Isenberg D. A., Nourooz-Zadeh J. Oxidative stress in systemic lupus erythematosus and allied conditions with vascular involvement. Rheumatology (Oxford) 1999 Jun;38(6):529–534. doi: 10.1093/rheumatology/38.6.529. [DOI] [PubMed] [Google Scholar]
- Arnett F. C., Edworthy S. M., Bloch D. A., McShane D. J., Fries J. F., Cooper N. S., Healey L. A., Kaplan S. R., Liang M. H., Luthra H. S. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum. 1988 Mar;31(3):315–324. doi: 10.1002/art.1780310302. [DOI] [PubMed] [Google Scholar]
- Basu S., Eriksson M. Lipid peroxidation induced by an early inflammatory response in endotoxaemia. Acta Anaesthesiol Scand. 2000 Jan;44(1):17–23. doi: 10.1034/j.1399-6576.2000.440104.x. [DOI] [PubMed] [Google Scholar]
- Basu S., Eriksson M. Oxidative injury and survival during endotoxemia. FEBS Lett. 1998 Nov 6;438(3):159–160. doi: 10.1016/s0014-5793(98)01290-3. [DOI] [PubMed] [Google Scholar]
- Basu S., Nozari A., Liu X. L., Rubertsson S., Wiklund L. Development of a novel biomarker of free radical damage in reperfusion injury after cardiac arrest. FEBS Lett. 2000 Mar 17;470(1):1–6. doi: 10.1016/s0014-5793(00)01279-5. [DOI] [PubMed] [Google Scholar]
- Basu S. Oxidative injury induced cyclooxygenase activation in experimental hepatotoxicity. Biochem Biophys Res Commun. 1999 Jan 27;254(3):764–767. doi: 10.1006/bbrc.1998.9956. [DOI] [PubMed] [Google Scholar]
- Basu S. Radioimmunoassay of 15-keto-13,14-dihydro-prostaglandin F2alpha: an index for inflammation via cyclooxygenase catalysed lipid peroxidation. Prostaglandins Leukot Essent Fatty Acids. 1998 May;58(5):347–352. doi: 10.1016/s0952-3278(98)90070-9. [DOI] [PubMed] [Google Scholar]
- Basu S. Radioimmunoassay of 8-iso-prostaglandin F2alpha: an index for oxidative injury via free radical catalysed lipid peroxidation. Prostaglandins Leukot Essent Fatty Acids. 1998 Apr;58(4):319–325. doi: 10.1016/s0952-3278(98)90042-4. [DOI] [PubMed] [Google Scholar]
- Blake D. R., Merry P., Unsworth J., Kidd B. L., Outhwaite J. M., Ballard R., Morris C. J., Gray L., Lunec J. Hypoxic-reperfusion injury in the inflamed human joint. Lancet. 1989 Feb 11;1(8633):289–293. doi: 10.1016/s0140-6736(89)91305-6. [DOI] [PubMed] [Google Scholar]
- Evans P. J., Cecchini R., Halliwell B. Oxidative damage to lipids and alpha 1-antiproteinase by phenylbutazone in the presence of haem proteins: protection by ascorbic acid. Biochem Pharmacol. 1992 Sep 1;44(5):981–984. doi: 10.1016/0006-2952(92)90131-2. [DOI] [PubMed] [Google Scholar]
- Fu J. Y., Masferrer J. L., Seibert K., Raz A., Needleman P. The induction and suppression of prostaglandin H2 synthase (cyclooxygenase) in human monocytes. J Biol Chem. 1990 Oct 5;265(28):16737–16740. [PubMed] [Google Scholar]
- Gutteridge J. M. Bleomycin-detectable iron in knee-joint synovial fluid from arthritic patients and its relationship to the extracellular antioxidant activities of caeruloplasmin, transferrin and lactoferrin. Biochem J. 1987 Jul 15;245(2):415–421. doi: 10.1042/bj2450415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutteridge J. M., Halliwell B. The measurement and mechanism of lipid peroxidation in biological systems. Trends Biochem Sci. 1990 Apr;15(4):129–135. doi: 10.1016/0968-0004(90)90206-q. [DOI] [PubMed] [Google Scholar]
- Halliwell B. Oxygen radicals, nitric oxide and human inflammatory joint disease. Ann Rheum Dis. 1995 Jun;54(6):505–510. doi: 10.1136/ard.54.6.505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirakata M., Suwa A., Takeda Y., Matsuoka Y., Irimajiri S., Targoff I. N., Hardin J. A., Craft J. Autoantibodies to glycyl-transfer RNA synthetase in myositis. Association with dermatomyositis and immunologic heterogeneity. Arthritis Rheum. 1996 Jan;39(1):146–151. doi: 10.1002/art.1780390119. [DOI] [PubMed] [Google Scholar]
- Humad S., Zarling E., Clapper M., Skosey J. L. Breath pentane excretion as a marker of disease activity in rheumatoid arthritis. Free Radic Res Commun. 1988;5(2):101–106. doi: 10.3109/10715768809066917. [DOI] [PubMed] [Google Scholar]
- Kaur H., Edmonds S. E., Blake D. R., Halliwell B. Hydroxyl radical generation by rheumatoid blood and knee joint synovial fluid. Ann Rheum Dis. 1996 Dec;55(12):915–920. doi: 10.1136/ard.55.12.915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meagher E. A., Barry O. P., Burke A., Lucey M. R., Lawson J. A., Rokach J., FitzGerald G. A. Alcohol-induced generation of lipid peroxidation products in humans. J Clin Invest. 1999 Sep;104(6):805–813. doi: 10.1172/JCI5584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrow J. D., Awad J. A., Kato T., Takahashi K., Badr K. F., Roberts L. J., 2nd, Burk R. F. Formation of novel non-cyclooxygenase-derived prostanoids (F2-isoprostanes) in carbon tetrachloride hepatotoxicity. An animal model of lipid peroxidation. J Clin Invest. 1992 Dec;90(6):2502–2507. doi: 10.1172/JCI116143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrow J. D., Hill K. E., Burk R. F., Nammour T. M., Badr K. F., Roberts L. J., 2nd A series of prostaglandin F2-like compounds are produced in vivo in humans by a non-cyclooxygenase, free radical-catalyzed mechanism. Proc Natl Acad Sci U S A. 1990 Dec;87(23):9383–9387. doi: 10.1073/pnas.87.23.9383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrow J. D., Roberts L. J., 2nd The isoprostanes. Current knowledge and directions for future research. Biochem Pharmacol. 1996 Jan 12;51(1):1–9. doi: 10.1016/0006-2952(95)02072-1. [DOI] [PubMed] [Google Scholar]
- Reilly M., Delanty N., Lawson J. A., FitzGerald G. A. Modulation of oxidant stress in vivo in chronic cigarette smokers. Circulation. 1996 Jul 1;94(1):19–25. doi: 10.1161/01.cir.94.1.19. [DOI] [PubMed] [Google Scholar]
- Robinson J., Watson F., Bucknall R. C., Edwards S. W. Activation of neutrophil reactive-oxidant production by synovial fluid from patients with inflammatory joint disease. Soluble and insoluble immunoglobulin aggregates activate different pathways in primed and unprimed cells. Biochem J. 1992 Sep 1;286(Pt 2):345–351. doi: 10.1042/bj2860345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowley D., Gutteridge J. M., Blake D., Farr M., Halliwell B. Lipid peroxidation in rheumatoid arthritis: thiobarbituric acid-reactive material and catalytic iron salts in synovial fluid from rheumatoid patients. Clin Sci (Lond) 1984 Jun;66(6):691–695. doi: 10.1042/cs0660691. [DOI] [PubMed] [Google Scholar]
- Stevens C. R., Benboubetra M., Harrison R., Sahinoglu T., Smith E. C., Blake D. R. Localisation of xanthine oxidase to synovial endothelium. Ann Rheum Dis. 1991 Nov;50(11):760–762. doi: 10.1136/ard.50.11.760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vane J. R., Botting R. M. A better understanding of anti-inflammatory drugs based on isoforms of cyclooxygenase (COX-1 and COX-2). Adv Prostaglandin Thromboxane Leukot Res. 1995;23:41–48. [PubMed] [Google Scholar]
- Xie W. L., Chipman J. G., Robertson D. L., Erikson R. L., Simmons D. L. Expression of a mitogen-responsive gene encoding prostaglandin synthase is regulated by mRNA splicing. Proc Natl Acad Sci U S A. 1991 Apr 1;88(7):2692–2696. doi: 10.1073/pnas.88.7.2692. [DOI] [PMC free article] [PubMed] [Google Scholar]