Abstract
OBJECTIVES—Examination of synovial tissue (ST) obtained at surgery because of end stage destructive rheumatoid arthritis (RA) showed that macrophages and fibroblasts are the major cell types at the cartilage-pannus junction (CPJ). This study aimed at defining the cell infiltrate and mediators of joint destruction in ST selected at arthroscopy from the CPJ in patients with RA who did not require joint surgery. METHODS—Paired synovial biopsy specimens were obtained at arthroscopy from ST adjacent to the CPJ and the suprapatellar pouch from the knee joints of 17 patients with RA. Immunohistological analysis was performed using monoclonal antibodies to detect T cells, B cells, plasma cells, macrophages, fibroblast-like synoviocytes, mast cells, and granzyme B+ cytotoxic cells as well as the expression of metalloproteinase (MMP)-1, MMP-3, and MMP-13. The sections were evaluated by computer assisted image analysis and semiquantitative analysis. RESULTS—The cell infiltrate comprised mainly T cells, macrophages, and plasma cells. The ST was also infiltrated by the other cell types, but at lower numbers. Expression of MMPs was abundant, especially MMP-3. The features of ST at the CPJ were generally similar to those at the suprapatellar pouch. CONCLUSIONS—The synovium at the CPJ in patients with RA who did not require joint surgery exhibits, in general, the same type of cell infiltrate and expression of MMPs and granzymes as ST from the suprapatellar pouch. The pathological changes that have been described at the CPJ in patients with RA with end stage, destructive disease may well reflect the transition to a process in which macrophages, fibroblast-like synoviocytes, and other cell types become increasingly important.
Full Text
The Full Text of this article is available as a PDF (143.5 KB).
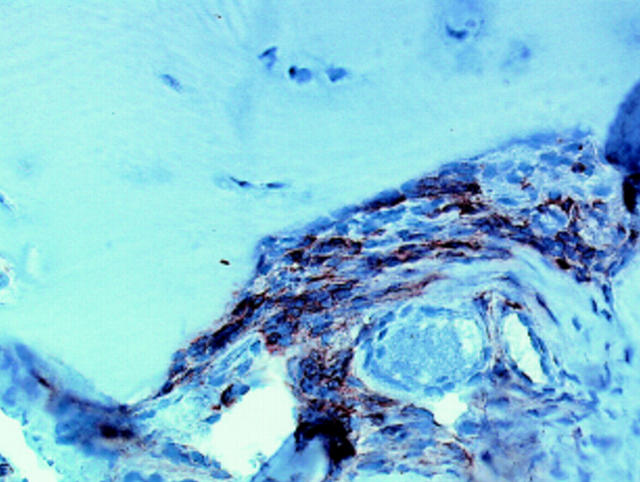
Figure 1 .
CD3 positive T cells localised at the cartilage-pannus junction in arthroscopically derived rheumatoid synovial tissue. Single stain peroxidase technique; Mayer's haemalum counterstained.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnett F. C., Edworthy S. M., Bloch D. A., McShane D. J., Fries J. F., Cooper N. S., Healey L. A., Kaplan S. R., Liang M. H., Luthra H. S. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum. 1988 Mar;31(3):315–324. doi: 10.1002/art.1780310302. [DOI] [PubMed] [Google Scholar]
- Aupperle K. R., Boyle D. L., Hendrix M., Seftor E. A., Zvaifler N. J., Barbosa M., Firestein G. S. Regulation of synoviocyte proliferation, apoptosis, and invasion by the p53 tumor suppressor gene. Am J Pathol. 1998 Apr;152(4):1091–1098. [PMC free article] [PubMed] [Google Scholar]
- Bromley M., Fisher W. D., Woolley D. E. Mast cells at sites of cartilage erosion in the rheumatoid joint. Ann Rheum Dis. 1984 Feb;43(1):76–79. doi: 10.1136/ard.43.1.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bromley M., Woolley D. E. Histopathology of the rheumatoid lesion. Identification of cell types at sites of cartilage erosion. Arthritis Rheum. 1984 Aug;27(8):857–863. doi: 10.1002/art.1780270804. [DOI] [PubMed] [Google Scholar]
- Chu C. Q., Field M., Allard S., Abney E., Feldmann M., Maini R. N. Detection of cytokines at the cartilage/pannus junction in patients with rheumatoid arthritis: implications for the role of cytokines in cartilage destruction and repair. Br J Rheumatol. 1992 Oct;31(10):653–661. doi: 10.1093/rheumatology/31.10.653. [DOI] [PubMed] [Google Scholar]
- Dayer J. M., de Rochemonteix B., Burrus B., Demczuk S., Dinarello C. A. Human recombinant interleukin 1 stimulates collagenase and prostaglandin E2 production by human synovial cells. J Clin Invest. 1986 Feb;77(2):645–648. doi: 10.1172/JCI112350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deleuran B. W., Chu C. Q., Field M., Brennan F. M., Katsikis P., Feldmann M., Maini R. N. Localization of interleukin-1 alpha, type 1 interleukin-1 receptor and interleukin-1 receptor antagonist in the synovial membrane and cartilage/pannus junction in rheumatoid arthritis. Br J Rheumatol. 1992 Dec;31(12):801–809. doi: 10.1093/rheumatology/31.12.801. [DOI] [PubMed] [Google Scholar]
- Firestein G. S. Invasive fibroblast-like synoviocytes in rheumatoid arthritis. Passive responders or transformed aggressors? Arthritis Rheum. 1996 Nov;39(11):1781–1790. doi: 10.1002/art.1780391103. [DOI] [PubMed] [Google Scholar]
- Firestein G. S., Nguyen K., Aupperle K. R., Yeo M., Boyle D. L., Zvaifler N. J. Apoptosis in rheumatoid arthritis: p53 overexpression in rheumatoid arthritis synovium. Am J Pathol. 1996 Dec;149(6):2143–2151. [PMC free article] [PubMed] [Google Scholar]
- Froelich C. J., Zhang X., Turbov J., Hudig D., Winkler U., Hanna W. L. Human granzyme B degrades aggrecan proteoglycan in matrix synthesized by chondrocytes. J Immunol. 1993 Dec 15;151(12):7161–7171. [PubMed] [Google Scholar]
- Jasser M. Z., Mitchell P. G., Cheung H. S. Induction of stromelysin-1 and collagenase synthesis in fibrochondrocytes by tumor necrosis factor-alpha. Matrix Biol. 1994 Apr;14(3):241–249. doi: 10.1016/0945-053x(94)90188-0. [DOI] [PubMed] [Google Scholar]
- Kirkham B., Portek I., Lee C. S., Stavros B., Lenarczyk A., Lassere M., Edmonds J. Intraarticular variability of synovial membrane histology, immunohistology, and cytokine mRNA expression in patients with rheumatoid arthritis. J Rheumatol. 1999 Apr;26(4):777–784. [PubMed] [Google Scholar]
- Kobayashi I., Ziff M. Electron microscopic studies of the cartilage-pannus junction in rheumatoid arthritis. Arthritis Rheum. 1975 Sep-Oct;18(5):475–483. doi: 10.1002/art.1780180507. [DOI] [PubMed] [Google Scholar]
- Kraan M. C., Haringman J. J., Ahern M. J., Breedveld F. C., Smith M. D., Tak P. P. Quantification of the cell infiltrate in synovial tissue by digital image analysis. Rheumatology (Oxford) 2000 Jan;39(1):43–49. doi: 10.1093/rheumatology/39.1.43. [DOI] [PubMed] [Google Scholar]
- Kraan M. C., Versendaal H., Jonker M., Bresnihan B., Post W. J., t Hart B. A., Breedveld F. C., Tak P. P. Asymptomatic synovitis precedes clinically manifest arthritis. Arthritis Rheum. 1998 Aug;41(8):1481–1488. doi: 10.1002/1529-0131(199808)41:8<1481::AID-ART19>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- Kullmann F., Judex M., Neudecker I., Lechner S., Jüsten H. P., Green D. R., Wessinghage D., Firestein G. S., Gay S., Schölmerich J. Analysis of the p53 tumor suppressor gene in rheumatoid arthritis synovial fibroblasts. Arthritis Rheum. 1999 Aug;42(8):1594–1600. doi: 10.1002/1529-0131(199908)42:8<1594::AID-ANR5>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- Mills K. Pathology of the knee joint in rheumatoid arthritis. A contribution to the understanding of synovectomy. J Bone Joint Surg Br. 1970 Nov;52(4):746–756. [PubMed] [Google Scholar]
- Mohr W., Menninger H. Polymorphonuclear granulocytes at the pannus-cartilage junction in rheumatoid arthritis. Arthritis Rheum. 1980 Dec;23(12):1413–1414. doi: 10.1002/art.1780231224. [DOI] [PubMed] [Google Scholar]
- Mort J. S., Dodge G. R., Roughley P. J., Liu J., Finch S. J., DiPasquale G., Poole A. R. Direct evidence for active metalloproteinases mediating matrix degradation in interleukin 1-stimulated human articular cartilage. Matrix. 1993 Mar;13(2):95–102. doi: 10.1016/s0934-8832(11)80068-5. [DOI] [PubMed] [Google Scholar]
- Muirden K. D. Electron microscopic studies of the synovial-cartilage junction in rheumatoid arthritis. Eur J Rheumatol Inflamm. 1982;5(1):30–38. [PubMed] [Google Scholar]
- Shiozawa S., Shiozawa K., Fujita T. Morphologic observations in the early phase of the cartilage-pannus junction. Light and electron microscopic studies of active cellular pannus. Arthritis Rheum. 1983 Apr;26(4):472–478. doi: 10.1002/art.1780260404. [DOI] [PubMed] [Google Scholar]
- Tak P. P., Kummer J. A., Hack C. E., Daha M. R., Smeets T. J., Erkelens G. W., Meinders A. E., Kluin P. M., Breedveld F. C. Granzyme-positive cytotoxic cells are specifically increased in early rheumatoid synovial tissue. Arthritis Rheum. 1994 Dec;37(12):1735–1743. doi: 10.1002/art.1780371205. [DOI] [PubMed] [Google Scholar]
- Tak P. P., Smeets T. J., Boyle D. L., Kraan M. C., Shi Y., Zhuang S., Zvaifler N. J., Breedveld F. C., Firestein G. S. p53 overexpression in synovial tissue from patients with early and longstanding rheumatoid arthritis compared with patients with reactive arthritis and osteoarthritis. Arthritis Rheum. 1999 May;42(5):948–953. doi: 10.1002/1529-0131(199905)42:5<948::AID-ANR13>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- Tak P. P., Smeets T. J., Daha M. R., Kluin P. M., Meijers K. A., Brand R., Meinders A. E., Breedveld F. C. Analysis of the synovial cell infiltrate in early rheumatoid synovial tissue in relation to local disease activity. Arthritis Rheum. 1997 Feb;40(2):217–225. doi: 10.1002/art.1780400206. [DOI] [PubMed] [Google Scholar]
- Tak P. P., Spaeny-Dekking L., Kraan M. C., Breedveld F. C., Froelich C. J., Hack C. E. The levels of soluble granzyme A and B are elevated in plasma and synovial fluid of patients with rheumatoid arthritis (RA). Clin Exp Immunol. 1999 May;116(2):366–370. doi: 10.1046/j.1365-2249.1999.00881.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tak P. P., Zvaifler N. J., Green D. R., Firestein G. S. Rheumatoid arthritis and p53: how oxidative stress might alter the course of inflammatory diseases. Immunol Today. 2000 Feb;21(2):78–82. doi: 10.1016/s0167-5699(99)01552-2. [DOI] [PubMed] [Google Scholar]
- Tak P. P., van der Lubbe P. A., Cauli A., Daha M. R., Smeets T. J., Kluin P. M., Meinders A. E., Yanni G., Panayi G. S., Breedveld F. C. Reduction of synovial inflammation after anti-CD4 monoclonal antibody treatment in early rheumatoid arthritis. Arthritis Rheum. 1995 Oct;38(10):1457–1465. doi: 10.1002/art.1780381012. [DOI] [PubMed] [Google Scholar]
- Tateishi H. Ultrastructure of synovio-cartilage junction in rheumatoid arthritis. Kobe J Med Sci. 1973 Jun;19(2):51–66. [PubMed] [Google Scholar]
- Tetlow L. C., Woolley D. E. Distribution, activation and tryptase/chymase phenotype of mast cells in the rheumatoid lesion. Ann Rheum Dis. 1995 Jul;54(7):549–555. doi: 10.1136/ard.54.7.549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tetlow L. C., Woolley D. E. Mast cells, cytokines, and metalloproteinases at the rheumatoid lesion: dual immunolocalisation studies. Ann Rheum Dis. 1995 Nov;54(11):896–903. doi: 10.1136/ard.54.11.896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolley D. E., Crossley M. J., Evanson J. M. Collagenase at sites of cartilage erosion in the rheumatoid joint. Arthritis Rheum. 1977 Jul-Aug;20(6):1231–1239. doi: 10.1002/art.1780200612. [DOI] [PubMed] [Google Scholar]
- Woolley D. E., Tetlow L. C. Observations on the microenvironmental nature of cartilage degradation in rheumatoid arthritis. Ann Rheum Dis. 1997 Mar;56(3):151–161. doi: 10.1136/ard.56.3.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youssef P. P., Kraan M., Breedveld F., Bresnihan B., Cassidy N., Cunnane G., Emery P., Fitzgerald O., Kane D., Lindblad S. Quantitative microscopic analysis of inflammation in rheumatoid arthritis synovial membrane samples selected at arthroscopy compared with samples obtained blindly by needle biopsy. Arthritis Rheum. 1998 Apr;41(4):663–669. doi: 10.1002/1529-0131(199804)41:4<663::AID-ART13>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- Youssef P. P., Smeets T. J., Bresnihan B., Cunnane G., Fitzgerald O., Breedveld F., Tak P. P. Microscopic measurement of cellular infiltration in the rheumatoid arthritis synovial membrane: a comparison of semiquantitative and quantitative analysis. Br J Rheumatol. 1998 Sep;37(9):1003–1007. doi: 10.1093/rheumatology/37.9.1003. [DOI] [PubMed] [Google Scholar]
- Youssef P., Roth J., Frosch M., Costello P., Fitzgerald O., Sorg C., Bresnihan B. Expression of myeloid related proteins (MRP) 8 and 14 and the MRP8/14 heterodimer in rheumatoid arthritis synovial membrane. J Rheumatol. 1999 Dec;26(12):2523–2528. [PubMed] [Google Scholar]