Abstract
The mitochondrial DNA (kinetoplast DNA) of the trypanosomatid Crithidia fasciculata has an unusual structure composed of minicircles and maxicircles topologically interlocked into a single network and organized in a disc-shaped structure at the base of the flagellum. We previously purified a structure-specific endonuclease (SSE1), based on its RNase H activity, that is enriched in isolated kinetoplasts. The endonuclease gene has now been cloned, sequenced, and found to be closely related to the 5′ exonuclease domain of bacterial DNA polymerase I proteins. Although the protein does not contain a typical mitochondrial leader sequence, the enzyme is shown to colocalize with a type II DNA topoisomerase and a DNA polymerase β at antipodal sites flanking the kinetoplast disc. Cell synchronization studies with an epitope-tagged construct show that the localization of the endonuclease to the antipodal sites varies in a cell cycle-dependent manner similar to that of the DNA polymerase β [Johnson, C. E. & Englund, P. T. (1998) J. Cell Biol. 143, 911–919]. Immunofluorescent localization of SSE1 to the antipodal sites is only observed during kinetoplast replication. Together, these results suggest a point of control for kinetoplast DNA replication through the regulation of the availability of DNA replication proteins and a possible role for the antipodal sites in removal of RNA primers and the repair of gaps in newly replicated minicircles.
Keywords: trypanosome, ribonuclease H, mitochondria
The trypanosomatid Crithidia fasciculata has an unusual mitochondrial DNA structure called the kinetoplast DNA, which is composed of 5,000 minicircles of 2.5 kilobases (kb) and 25 maxicircles of 37 kb catenated into a single network. The maxicircles encode conventional mitochondrial proteins and ribosomal RNAs. Unlike most mitochondrial DNA, kinetoplast DNA replicates only once in the cell cycle in approximate synchrony with nuclear DNA replication (1, 2). Minicircles replicate free of the network as θ intermediates (3). Light-strand synthesis is RNA primed at each of the two universal minicircle sequences and occurs continuously (4–6). Heavy-strand synthesis occurs discontinuously via Okazaki-like fragments that are also likely to be RNA primed (5, 7). Nicks and gaps remaining in newly replicated minicircles are partially repaired before reattachment to the network but are not completely repaired until just before network division into two daughter networks. (4, 5, 8, 9).
Several enzymes involved in kinetoplast DNA replication have been purified and localized within the kinetoplast. A primase has been localized to a region adjacent to the two faces of the kinetoplast disk, suggesting that RNA priming and the early stages of minicircle replication may occur in this region (10). Additionally, a topoisomerase II and a DNA polymerase β have been localized to two antipodal sites flanking the kinetoplast disc where the minicircles are reattached to the network (11–14). Minicircle replication intermediates have also been detected at these sites (11). The low processivity of polymerase β suggests that it may be involved in the repair of gaps remaining in newly replicated minicircles. The topoisomerase II is likely to mediate minicircle reattachment after partial gap repair.
To further define the components of the replication machinery and their localization relative to the kinetoplast disc, we have sought to identify and purify additional kinetoplast replication proteins. The purification and characterization of a structure-specific endonuclease (SSE1) enriched in the kinetoplast fraction of C. fasciculata was reported previously (15). SSE1 cleaves endonucleolytically at branched DNA structures having an unannealed 5′ tail. The enzyme also possesses 5′ exonuclease activity and RNase H activity and may play a role in the processing of RNA primers on nascent minicircles. Here we report the cloning and sequencing of SSE1 and its cell cycle-dependent localization. Like the β polymerase, SSE1 localizes to the antipodal sites flanking the kinetoplast disk only during kinetoplast DNA replication.
MATERIALS AND METHODS
Gene Cloning.
Tryptic digestion and peptide sequencing of SSE1 were performed by the Harvard Microchemistry Facility. Degenerate primers were synthesized based on the peptide sequences and used in PCR reactions to clone a portion of the SSE1 coding region. Reactions consisted of 100 ng of C. fasciculata genomic DNA, 200 μM dNTPs, 5.0% DMSO, 3 mM MgCl2, 0.5 μM oligonucleotides, 20 mM Tris⋅HCl (pH 8.4), 50 mM KCl, and 2 units of Taq polymerase. Thirty-three rounds of PCR with a 50°C annealing temperature were performed. The oligonucleotides used were 5′-TTCATVCCVGGRTTRTTRTC-3′ and 5′-GCNCTYSSTTYGAYCC-3′. These oligonucleotides were chosen based on peptide sequences (DNIPGMK and AHSFDP) and the C. fasciculata codon bias. A 531-bp product was cloned into pGEM 7 (Promega) and sequenced. The PCR product was used to screen a λ GEM11 C. fasciculata genomic library (16). Individual clones were used directly for cycle sequencing by using an Applied Biosystems automated sequencer at the UCLA DNA Sequencing Facility.
Recombinant SSE1 Expression in C. fasciculata.
A 2.6-kb SphI fragment containing SSE1 coding and 5′ flanking sequence was cloned into pGEM 7. A NotI site was engineered into the carboxyl terminus of SSE1 just prior to the stop codon by oligonucleotide mutagenesis by using a Promega Gene Editor kit and the oligonucleotide 5′-GTGCGAGCCTTTCCTCAACAAGCGGCCGCTGTGAGAGGGAAGAGAGGTGGGACTG-3′ according to the manufacturer’s directions. Three copies of the influenza hemagglutinin epitope (HA3) were cloned into the NotI site. The hygromycin phosphotransferase gene flanked by 5′ and 3′ untranslated regions from Leishmanina major dihydrofolate reductase–thymidylate synthase was cloned downstream of the HA3-tagged SSE1 gene. C. fasciculata cells were transformed and selected as described (2). Cells expressing SSE1–HA3 were synchronized as described (2).
Immunolocalization.
All steps were performed at room temperature unless otherwise stated, and a humid chamber was used for all incubations. Cells (2 × 106) in PBS were spotted on a poly-l-lysine-coated slide and allowed to adhere for 30 min. The cells were fixed in 4.0% paraformaldehyde in PBS for 2.5 min. Fixation was stopped by two 5-min washes in 0.1 M glycine in PBS. Slides were incubated for 5 min in 0.25% Triton X-100 in PBS, washed twice for 5 min in PBS, and then placed in methanol at −20°C overnight. After washing 3 times in PBS for 5 min to rehydrate the cells, the cells were blocked for 30 min at 37°C in 20% goat serum (GS) in PBST (PBS + 0.05% Tween 20). After blocking, the cells were incubated with a 1:300 dilution of 12CA5 antibody (Babco, Richmond, CA) in 20% GS in PBST for 45 min at 37°C. Three 5-min washes in PBST were followed by incubation with a 1:50 dilution of goat anti-mouse IgG–FITC (Sigma) in 20% GS in PBST for 30 min at 37°C. The slides were washed again 3 times for 5 min in PBST, stained for 3 min with 0.1 μg/ml 4′,6-diamidino-2-phenylindole (DAPI) in PBST, rinsed with water, and mounted in Prolong (Molecular Probes).
The slides with double immunolocalizations were washed twice in TNT (0.1 M Tris⋅HCl, pH 7.5/0.15 M NaCl/0.05% Tween 20) for 5 min and blocked in 20% GS in TNT at 37°C for 1 hr. The cells were then incubated with either 1:100 dilution of rabbit anti-topoisomerase II (17) or 1:50 dilution of rabbit anti-polymerase β (11) in 20% GS in TNT for 45 min at 37°C. Excess primary antibody was removed by three 5-min washes in TNT, and the cells were blocked again in TNB (0.1 M Tris⋅HCl, pH 7.5/0.15 M NaCl/0.5% Dupont blocking reagent) for 30 min. The final antibody was a 1:300 dilution of goat anti-rabbit-horseradish peroxidase (Sigma) in TNB for 30 min. The slides were washed three more times in TNT and 150 μl of a 1:100 dilution of tetramethylrhodamine tyramide in amplification buffer [Renaissance-Direct (Red)] was added to the cells for 10 min. Finally, the slides were washed again in TNT three times, stained with DAPI, and mounted as above.
Recombinant Protein Expression.
SSE1 coding region was cloned into a pET-30 LIC vector (Novagen) by PCR using oligonucleotides E28: 5′-GACGACGACAAGATGGCGCTGCGAGAGAGAGCG-3′ and E29: 5′-GAGGAGAAGCCCGGTTCACTTGTTGAGGAAAGGCTC-3′ following the manufacturer’s protocol. pET-30 LIC-SSE1 expresses SSE1 with an amino-terminal 6-histidine tag followed by an S-binding tag. Escherichia coli BL21D3 cells transformed with the recombinant plasmid were grown in SOB (2% Bacto tryptone/0.5% yeast extract/0.05% NaCl/2.5 mM KCl/10 mM MgCl2) (18) supplemented with 30 μg/ml kanamycin at 30°C until an A595 of 0.7 and then induced with 0.2 mM isopropyl β-d-thiogalactoside (IPTG) for 2.5 hr. Cells were harvested and stored at −70°C. The recombinant protein was isolated by nickel chelate chromatography with Novagen His⋅Bind resin and dialyzed twice for 30 min against 500 ml of each of the following buffers: buffer 1 (20% glycerol/0.2 M imidazole/50 mM sodium phosphate, pH 6.5/0.5 M NaCl/2.5 mM 2-mercaptoethanol); buffer 2 (20% glycerol/50 mM sodium phosphate, pH 6.5/0.35 M NaCl/20 mM MgCl2/2.5 mM 2-mercaptoethanol); buffer 3 (20% glycerol/50 mM sodium phosphate, pH 6.5/0.2M NaCl/2 mM EDTA/2.5 mM 2-mercaptoethanol). The activity of the recombinant protein was assayed by using endonuclease and RNase H assays as described (15) except that a M13 DNA—[32P]RNA substrate was used instead of a poly([32P]rA⋅poly(dT)) substrate. The M13 DNA–[32P]RNA substrate was synthesized in a reaction containing 40 mM Tris⋅HCl, pH 8.0/10 mM MgCl2/5 mM DTT/50 mM KCl/50 μg/ml BSA/2μg of M13 mp18 DNA/300 μM GTP, CTP, and UTP/1μM ATP/[α-32P]ATP (800 Ci/mmol; 1 Ci = 37 GBq)/9 units of E. coli RNA polymerase.
5′ Splice Acceptor Site.
Whole-cell RNA was isolated by using Triazol (GIBCO/BRL) according to the manufacturer’s instructions and poly(A)+ RNA was isolated with a poly(A) RNA isolation kit (United States Biochemica) also according to the manufacturer’s protocol. cDNA was synthesized with Moloney–murine leukemia virus reverse transcriptase and E18: 5′-TGGCGGGCTCGAAAAGCAAG-3′ at nucleotide position 1,102–1,083. The cDNA was amplified with miniexon: 5′-AACGCTATATAAGTATCAGTTTCTGTACTT-3′ and E27: 5′-GCCGTACTTCTTCAGAAGCTG-3′ at nucleotide position 834–814. The PCR reaction products were used in further reaction with miniexon and E36: 5′-CAGTCTTGAGCCGCGCATC-3′ at nucleotide position 529–511. The product was cloned and sequenced to determine the 5′ splice acceptor site.
RESULTS
Gene Cloning.
SSE1 was cloned by PCR amplification of C. fasciculata genomic DNA using degenerate primers based on peptide sequence data obtained by microsequencing of tryptic peptides. A PCR fragment was used to screen a λ C. fasciculata genomic library, and several positive clones were sequenced. The 5′ splice acceptor site was determined by reverse transcriptase PCR using miniexon and internal primers. The sequence of SSE1 is shown in Fig. 1. Unlike polymerase β, SSE1 does not contain a typical cleavable presequence for mitochondrial import (19). The ORF for SSE1 is predicted to encode a protein of 33.5 kDa, very close to the 32.4 kDa determined for the purified protein (15).
Figure 1.
Sequence of SSE1. The spliced leader (miniexon) is indicated in bold. The peptides obtained from microsequencing are underlined. ∗ indicates a stop codon.
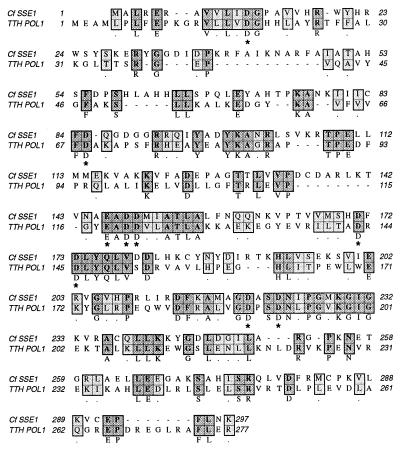
Based on its enzymatic characteristics, SSE1 initially appeared to be a member of the FEN family of eukaryotic structure-specific endonucleases, but sequence analysis indicates that SSE1 is more closely related to the 5′ → 3′ exonuclease of bacterial DNA polymerase I enzymes, an activity more accurately described as a structure-specific endonuclease. A blast program analysis of the SSE1 coding region reveals SSE1 is most similar to the 5′ → 3′ exonuclease regions of DNA polymerase I from several bacterial species. By using the clustal w alignment program, the amino-terminal domain of Tth DNA polymerase I shows the highest similarity to SSE1, with 29% identical residues and 16% similar residues. SSE1 is only 15% identical and 19% similar to mouse FEN1. The alignment of SSE1 and Tth DNA polymerase I is shown in Fig. 2. The first 300 aa of the TTH DNA polymerase encode the 5′ → 3′ exonuclease region of the protein. Amino acid residues marked with an asterisk are likely to be involved in either structure specific endonuclease or RNase H activity based on mutational analysis of E. coli DNA polymerase I, human FEN1, and T4 RNase H (20–22).
Figure 2.
Similarity of SSE1 to the 5′exonuclease domain of Thermus thermophilus (TTH) polymerase I. SSE1 was aligned to the first 277 aa of Tth polymerase I by using the clustal w program. Identities are shown in dark gray and similarities in light gray. The residues marked by ∗ are conserved among 18 bacterial and phage 5′ → 3′ exonucleases and have been shown to be involved with substrate binding or catalysis (20).
Recombinant Protein.
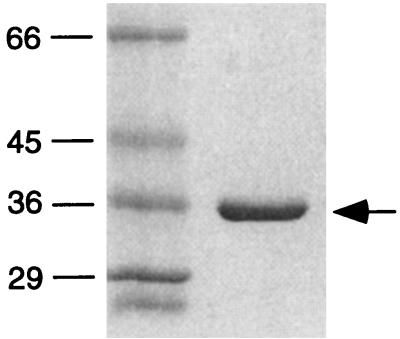
To characterize the SSE1 recombinant protein, the gene was cloned into a pET-30–LIC plasmid that placed a 6-histidine tag and an S-binding protein tag in the amino terminus of the protein. The recombinant protein was expressed in E. coli and purified by nickel chelate chromatography. The recombinant protein migrates as a single band on an SDS gel (Fig. 3). Although the protein was not present in inclusion bodies nor was it purified under denaturing conditions, the protein did not initially possess activity after purification. After dialysis in the presence of excess magnesium ion, the protein possessed both endonuclease activity and RNase H activity; however, the recombinant protein had a significantly lower specific activity than the native protein. The 5′ end-labeled substrate used in the endonuclease assays is diagramed in Fig. 4A. Like the purified protein, recombinant SSE1 cleaves the substrate in a structure-specific manner, releasing the intact 5′ tail of the substrate (Fig. 4B). The recombinant protein was also shown to have RNase H activity on a M13 DNA–[32P] RNA substrate (Fig. 4C).
Figure 3.
Purified recombinant SSE1. SDS gel electrophoresis of purified recombinant SSE1. Sizes of molecular mass markers are indicated in kDa. The arrow indicates recombinant SSE1.
Figure 4.
Recombinant SSE1 is active as both structure-specific endonuclease and RNase H. (A) Substrate for endonuclease assays. The 5′ 32P label is indicated by ∗. (B) Endonuclease assays with recombinant SSE1. Lane 1, no enzyme added; lane 2, 0.5 ng of enzyme; lane 3, 0.1 ng of enzyme; lane 4, 0.05 ng of enzyme; 21-nt product is indicated by an arrow. The size of the 21-nt product was determined by comparison to a sequencing ladder as in ref. 15. (C) RNase H assays with recombinant SSE1.
Immunolocalization.
SSE1 was initially purified based on its enrichment in kinetoplast extracts (15). An HA-tagged form of SSE1 was constructed to allow a precise intracellular localization of the nuclease. Three copies of the HA tag were cloned just upstream of the stop codon in SSE1 in a plasmid containing a hygromycin phosphotransferase gene for selection in C. fasciculata. The SSE1–HA3 protein was expressed in wild-type C. fasciculata cells and immunolocalized by using a monoclonal antibody to the HA tag. DAPI staining was used to visualize the nucleus (diffuse staining) and kinetoplast (bright staining, Fig. 5A). Immunolocalization of SSE1–HA3 shows two discrete sites flanking the kinetoplast (Fig. 5B).
Figure 5.
SSE1 has an antipodal kinetoplast localization. (A) DAPI staining of C. fasciculata cells transformed with a plasmid encoding SSE1–HA3; the kinetoplast is the brightly staining body and the nucleus is the diffusely staining body. (B) Immunofluorescence localization of SSE1–HA3. The kinetoplast is seen to be flanked by two bright dots following immunolocalization of the HA tag.
To further characterize the localization of the SSE1 nuclease, double-label immunofluorescence was used to localize SSE1–HA3 relative to topoisomerase II and β polymerase, both of which localize to the antipodal sites flanking the kinetoplast disc (11, 12). SSE1–HA3 colocalized (Fig. 6 B and E) with polymerase β (Fig. 6C) and also with topoisomerase II (Fig. 6F). The colocalization of these three replication proteins suggests that they may be components of an organized structure involved in kinetoplast duplication.
Figure 6.
Coimmunolocalization of SSE1–HA3 with polymerase β and topoisomerase II. (A) DAPI staining of C. fasciculata cells carrying SSE1–HA3; the brightly staining body is the kinetoplast. (B) The same cells as shown in A with immunolocalization of SSE1–HA3. (C) The same cells as shown in A with immunolocalization of polymerase β. (D) DAPI staining of C. fasciculata cells. (E) The same cells as shown in D with immunolocalization of SSE1–HA3. (F) The same cells as shown in D with immunolocalization of topoisomerase II.
Because SSE1 colocalizes with topoisomerase II and polymerase β, it was of interest to determine whether the localization of SSE1 varied over the cell cycle. Polymerase β has been shown to be present in the antipodal sites only during kinetoplast replication and is undetectable at other times in the cell cycle (23). In contrast, topoisomerase II can be visualized at all times during the cell cycle although it is not always localized to the two protein complexes (23). C. fasciculata cells expressing SSE1–HA3 were synchronized by using hydroxyurea block and analyzed by using immunofluorescence for the presence and localization of SSE1–HA3 at 30-min intervals after release from the block. SSE1 appears to follow much the same pattern as polymerase β; it is either present in the two antipodal sites or not detectable above the background in the cells. Fig. 7A shows that a high percentage of the cells have the antipodal localization immediately after release from the hydroxyurea block and again at 180–270 min after release, times corresponding to the first and second S phases in the synchronized cells (24). At 120 min, a high percentage of the cells were dividing, and only ≈10% of the cells showed the antipodal localization of SSE1 at this time. This periodic localization of SSE1 to the antipodal sites flanking the kinetoplast is essentially identical to that of the DNA polymerase β (23).
Figure 7.
Localization of SSE1–HA3 during the cell cycle. (A) □ indicates percentage of cells containing two nuclei as a measure of cell synchronization. Cells were synchronized by release from a hydroxyurea block as described (2). ▴ indicates percentage of cells showing an antipodal localization of SSE1–HA3. Samples were taken at 30-min intervals after release from a hydroxyurea block. (B) Cells from an asynchronous culture demonstrate both localization patterns. Left, two cells are shown with DAPI staining; the brightly staining bodies are the kinetoplasts. Right, the same cells were immunolocalized with antibody to SSE1–HA3; one cell shows an antipodal localization of SSE1–HA3, and the other cell shows a lack of fluorescence above background.
The difference between the two states of immunolocalization is illustrated by cells from an asynchronous culture. In Fig. 7B Left, two cells can be seen with DAPI stain; the bright staining is the kinetoplast, and the diffuse staining is the nucleus. However, in Fig. 7B Right, where the cells are examined for the localization of SSE1–HA3, only one cell shows the antipodal localization and the other cell does not show immunofluorescence above background. However, like polymerase β, the SSE1 protein is present throughout the cell cycle based on Western blots of synchronized cells (data not shown; ref. 23).
DISCUSSION
Several kinetoplast replication proteins have been found to have distinct localizations relative to the kinetoplast disc in the trypanosomatid C. fasciculata. A type II DNA topoisomerase, a DNA polymerase β, and the SSE1 nuclease described here all localize at antipodal sites at the edge of the kinetoplast disc (11, 12). A DNA primase and p21, a highly basic DNA-binding protein, are localized at opposite faces of the kinetoplast disc, and the H1 histone-like proteins p16, p17, and p18 are localized throughout the kinetoplast disc (10, 25, 26). In addition, minicircles replicated free of the kinetoplast DNA network are found as gapped molecules at the antipodal sites (11), with some of the newly replicated minicircles containing RNA at the 5′ side of the gaps (5). The SSE1 nuclease and the β polymerase are well suited for roles in the repair of these gaps, and their localization at the antipodal sites suggests that this later stage in the replication of individual minicircles occurs at these sites. In contrast, the localization of a DNA primase at the faces of the kinetoplast disc suggests that initiation of minicircle replication is spatially separated from the later stages of primer removal, gap filling, and rejoining to the network.
The specific sequences involved in the mitochondrial import and antipodal localization of SSE1 are unknown. The histone-like proteins p16, p17, p18 and p21 were all found to have a novel 9-aa cleavable presequence containing basic and hydrophobic residues and lacking acidic residues similar to the usual mitochondrial targeting sequences in other eukaryotes (25, 26). That these unusually short presequences play a similar role in trypanosomatids is supported by the finding that the first 14 aa of p16 fused to mouse dihydrofolate reductase is sufficient for the import and processing of the chimeric protein into trypanosomal mitochondria (27). Neither SSE1 nor the kinetoplast topoisomerase have such amino-terminal sequences, whereas polymerase β does have a similar 9-aa cleavable presequence (16, 19). Some mitochondrial precursor proteins lacking N-terminal targeting signals have been shown to be targeted to mitochondria by means of internal signals (28). Putative internal mitochondrial targeting signals in SSE1 and topoisomerase II must also operate in conjunction with whatever signals determine the antipodal localization of these proteins. These latter signals are likely to be dictated by requirements for specific interaction with other proteins and may be unique for each of the proteins localized to these sites.
SSE1 has both RNase H and structure-specific endonuclease activities, two activities shown in other eukaryotic systems to be necessary for the complete removal of RNA primers (29–32). In bacteria, both activities are catalyzed by the 5′ exo/endonuclease activity of the DNA polymerase I. Sequence comparisons indicate that SSE1 is much more closely related to the bacterial DNA polymerases than to the FEN family of structure-specific endonucleases. E. coli strains with inactivating mutations in the 5′ exonuclease domain of DNA polymerase I are not viable because of an inability to process Okazaki fragments (33). Eukaryotic DNA polymerases lack this 5′ exonuclease domain and require the activity of a separate structure-specific nuclease for this necessary function. SSE1 has a predicted molecular mass of 33.5 kDa, whereas DNA polymerase I from bacterial species typically has a molecular mass of ≈100 kDa. The molecular mass of SSE1 predicted from the DNA sequence of the gene is in close agreement with that observed for the purified enzyme, indicating that the enzyme is not a proteolytic product of a prokaryotic-like DNA polymerase. The possibility that this kinetoplast nuclease might normally be associated with a DNA polymerase remains to be investigated.
Another nuclease with a molecular mass similar to that of SSE1 has been purified from mitochondrial extracts of C. fasciculata (34). This 32-kDa protein appears to be different from SSE1. It degrades both single-stranded and double-stranded oligonucleotides and is present in two forms, either a 32-kDa monomer or as a multimer in association with a 56-kDa polypeptide (34). Degradation of a 5′ 32P-labeled oligonucleotide by the enzyme yields a ladder of products and ultimately yields the terminal nucleotide as a 5′ mononucleotide (34). Immunolocalization of this enzyme is inconclusive so far.
The periodic immunolocalization of SSE1 to the antipodal sites at the edge of the kinetoplast disc precisely parallels that of the DNA polymerase β. In both cases, a distinct localization at these sites is observed during S phase, but only background fluorescence is detected when the cells are dividing. Yet, both enzymes are present throughout the cell cycle as judged by Western blotting (data not shown; ref. 23). The possibility that certain replication proteins are recruited to large multiprotein complexes during kinetoplast DNA replication and dispersed elsewhere at undetectable levels during cell division might reflect an aspect of the regulation of kinetoplast DNA replication.
Acknowledgments
We thank Paul Englund for the gift of anti-polymerase β antibody and David Campbell for comments on the manuscript. This work was supported by National Institutes of Health Grant GM53254 to D.S.R. and U.S. Public Health Service National Research Service Award GM07185 to M.L.E.
ABBREVIATIONS
- SSE1
structure-specific endonuclease-1
- PBS
phosphate buffered saline
- GS
goat serum
- HA
hemagglutinin
- DAPI
4′,6-diamidino-2-phenylindole
Footnotes
Data deposition: The sequence reported in this paper has been deposited in the GenBank database (accession no. AF124228).
References
- 1.Cosgrove W B, Skeen M J. J Protozool. 1970;17:172–177. doi: 10.1111/j.1550-7408.1970.tb02350.x. [DOI] [PubMed] [Google Scholar]
- 2.Pasion S G, Brown G W, Brown L M, Ray D S. J Cell Sci. 1994;107:3515–3520. doi: 10.1242/jcs.107.12.3515. [DOI] [PubMed] [Google Scholar]
- 3.Shapiro T A, Englund P T. Annu Rev Microbiol. 1995;49:117–143. doi: 10.1146/annurev.mi.49.100195.001001. [DOI] [PubMed] [Google Scholar]
- 4.Birkenmeyer L, Ray D S. J Biol Chem. 1986;261:2362–2368. [PubMed] [Google Scholar]
- 5.Birkenmeyer L, Sugisaki H, Ray D S. J Biol Chem. 1987;262:2384–2392. [PubMed] [Google Scholar]
- 6.Kitchin P A, Klein V A, Englund P T. J Biol Chem. 1985;260:3844–3851. [PubMed] [Google Scholar]
- 7.Kitchin P A, Klein V A, Fein B I, Englund P T. J Biol Chem. 1984;259:15532–15539. [PubMed] [Google Scholar]
- 8.Ntambi J M, Shapiro T A, Ryan K A, Englund P T. J Biol Chem. 1986;261:11890–11895. [PubMed] [Google Scholar]
- 9.Perez-Morga D, Englund P T. J Cell Biol. 1993;123:1069–1079. doi: 10.1083/jcb.123.5.1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li C J, Englund P T. J Biol Chem. 1997;272:20787–20792. doi: 10.1074/jbc.272.33.20787. [DOI] [PubMed] [Google Scholar]
- 11.Ferguson M, Torri A F, Ward D C, Englund P T. Cell. 1992;70:621–629. doi: 10.1016/0092-8674(92)90431-b. [DOI] [PubMed] [Google Scholar]
- 12.Melendy T, Sheline C, Ray D S. Cell. 1988;55:1083–1088. doi: 10.1016/0092-8674(88)90252-8. [DOI] [PubMed] [Google Scholar]
- 13.Perez-Morga D L, Englund P T. Cell. 1993;74:703–711. doi: 10.1016/0092-8674(93)90517-t. [DOI] [PubMed] [Google Scholar]
- 14.Simpson A M, Simpson L. J Protozool. 1976;23:583–587. doi: 10.1111/j.1550-7408.1976.tb03846.x. [DOI] [PubMed] [Google Scholar]
- 15.Engel M L, Ray D S. Nucleic Acids Res. 1998;26:4733–4738. doi: 10.1093/nar/26.20.4733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pasion S G, Hines J C, Aebersold R, Ray D S. Mol Biochem Parasitol. 1992;50:57–67. doi: 10.1016/0166-6851(92)90244-e. [DOI] [PubMed] [Google Scholar]
- 17.Melendy T, Ray D S. J Biol Chem. 1989;264:1870–1876. [PubMed] [Google Scholar]
- 18.Sambrook J, Fritsch E F, Maniatis T. Molecular Cloning: A Laboratory Manual. 2nd Ed. Plainview, NY: Cold Spring Harbor Lab. Press; 1989. [Google Scholar]
- 19.Torri A F, Englund P T. J Biol Chem. 1995;270:1–3. doi: 10.1074/jbc.270.8.3495. [DOI] [PubMed] [Google Scholar]
- 20.Xu Y, Derbyshire V, Ng K, Sun X C, Grindley N D, Joyce C M. J Mol Biol. 1997;268:284–302. doi: 10.1006/jmbi.1997.0967. [DOI] [PubMed] [Google Scholar]
- 21.Mueser T C, Nossal N G, Hyde C C. Cell. 1996;85:1101–1112. doi: 10.1016/s0092-8674(00)81310-0. [DOI] [PubMed] [Google Scholar]
- 22.Shen B, Nolan J P, Sklar L A, Park M S. Nucleic Acids Res. 1997;25:3332–3338. doi: 10.1093/nar/25.16.3332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Johnson C E, Englund P T. J Cell Biol. 1998;143:911–919. doi: 10.1083/jcb.143.4.911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hines J C, Ray D S. Mol Biochem Parasitol. 1997;88:249–252. doi: 10.1016/s0166-6851(97)00076-5. [DOI] [PubMed] [Google Scholar]
- 25.Hines J C, Ray D S. Mol Biochem Parasitol. 1998;94:41–52. doi: 10.1016/s0166-6851(98)00048-6. [DOI] [PubMed] [Google Scholar]
- 26.Xu C W, Hines J C, Engel M L, Russell D G, Ray D S. Mol Cell Biol. 1996;16:564–576. doi: 10.1128/mcb.16.2.564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hausler T, Stierhof Y D, Blattner J, Clayton C. Eur J Cell Biol. 1997;73:240–251. [PubMed] [Google Scholar]
- 28.Folsch H, Guiard B, Neupert W, Stuart R A. EMBO J. 1996;15:479–487. [PMC free article] [PubMed] [Google Scholar]
- 29.Huang L, Kim Y, Turchi J J, Bambara R A. J Biol Chem. 1994;269:25922–25927. [PubMed] [Google Scholar]
- 30.Turchi J J, Huang L, Murante R S, Kim Y, Bambara R A. Proc Natl Acad Sci USA. 1994;91:9803–9807. doi: 10.1073/pnas.91.21.9803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Goulian M, Richards S H, Heard C J, Bigsby B M. J Biol Chem. 1990;265:18461–18471. [PubMed] [Google Scholar]
- 32.Ishimi Y, Claude A, Bullock P, Hurwitz J. J Biol Chem. 1988;263:19723–19733. [PubMed] [Google Scholar]
- 33.Uyemura D, Lehman I R. J Biol Chem. 1976;251:4078–4084. [PubMed] [Google Scholar]
- 34.Li C J, Hwa K Y, Englund P T. Nucleic Acids Res. 1995;23:4426–4433. [PMC free article] [PubMed] [Google Scholar]