Abstract
OBJECTIVES—To examine whether inhibition of NF-κB induces apoptosis of human synovial cells stimulated by tumour necrosis factor α (TNFα), interleukin 1β (IL1β), and anti-Fas monoclonal antibody (mAb). METHODS—The expression of proliferating cell nuclear antigen (PCNA), NF-κB, and the presence of apoptotic synovial cells were determined in synovial tissues. Apoptosis of cultured synovial cells was induced by inhibition of NF-κB nuclear translocation by Z-Leu-Leu-Leu-aldehyde (LLL-CHO). The activation of caspase-3 and expression of XIAP and cIAP2 in synovial cells in LLL-CHO induced apoptosis was also examined. RESULTS—Abundant PCNA+ synovial cells were found in rheumatoid arthritis (RA) synovial tissue, though a few apoptotic synovial cells were also detected in the RA synovial tissues. Nuclear NF-κB was expressed in RA synovial cells. Electrophoretic mobility shift assay showed that treatment of cells with TNFα or IL1β significantly stimulated nuclear NF-κB activity. A small number of apoptotic synovial cells expressing intracellular active caspase-3 were found after treatment of cells with LLL-CHO. Although treatment of RA synovial cells with TNFα or IL1β alone did not induce apoptosis, apoptosis induced by LLL-CHO and caspase-3 activation were clearly enhanced in TNFα or IL1β stimulated synovial cells compared with unstimulated synovial cells. Furthermore, induction of apoptosis of synovial cells with caspase-3 activation by anti-Fas mAb was clearly increased by LLL-CHO. The expression of cIAP2 and XIAP in synovial cells may not directly influence the sensitivity of synovial cells to apoptosis induced by LLL-CHO. CONCLUSION—The results suggest that NF-κB inhibition may be a potentially important therapeutic approach for RA by correcting the imbalance between apoptosis and proliferation of synovial cells in RA synovial tissue.
Full Text
The Full Text of this article is available as a PDF (207.4 KB).
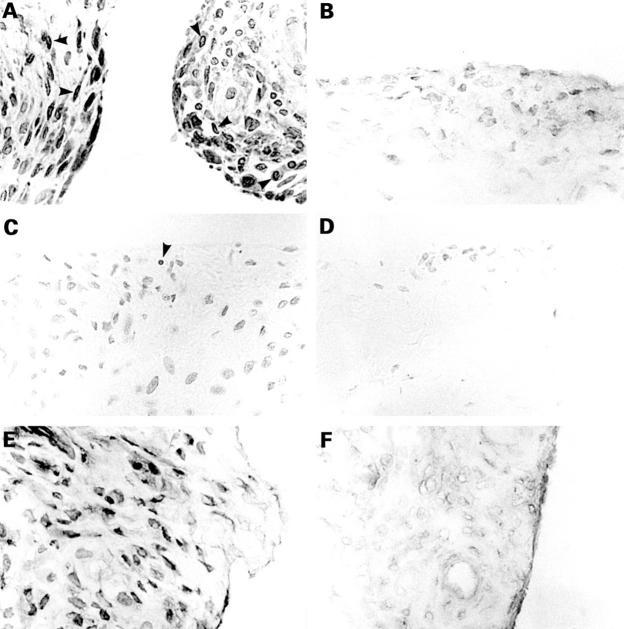
Figure 1 .
Expression of proliferating cell nuclear antigen (PCNA) (A, B), terminal deoxy (d)-UTP nick end labelling (TUNEL) assay (C, D), and NF-κB (E, F) in synovial tissues from patients with rheumatoid arthritis (RA) and osteoarthritis (OA). (A, B) PCNA staining in RA (A) and OA (B) synovial tissues. Note that the expression of PCNA was clearly detected in synovial cells of RA synovial tissue, but its expression was weak in OA synovial tissue. Arrowheads indicate representative PCNA+ cells. (C, D) TUNEL staining of RA (C) and OA (D) synovial tissues. A few synovial cells positive for TUNEL staining are present in RA synovial tissue, but those were rarely detected in OA synovial tissue. (E, F) NF-κB expression in RA (E) and OA (F) synovial tissue. Note the strong nuclear NF-κB expression in synovial cells of patients with RA. Nuclear NF-κB expression was not clear in synovial cells of patients with OA. Results shown are representative results from 10 patients with RA and 10 with OA. Note that no significant staining was observed by the use of control immunoglobulin (PCNA and NF-κB) or performed in the absence of biotinylated 16-dUTP (TUNEL) (data not shown). Magnification ×400.
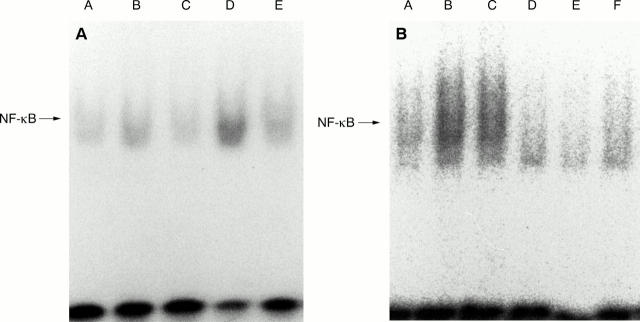
Figure 2 .
NF-κB nuclear translocation in cultured synovial cells determined by EMSA. (A) Note the presence of a weak basal NF-κB nuclear activity in unstimulated synovial cells, and markedly increased activity in cells stimulated by tumour necrosis factor α (TNFα) or interleukin 1β (IL1β). Synovial cells from patients with rheumatoid arthritis (RA) were cultured with or without TNFα (200 IU/ml) or IL1β (20 IU/ml). After cultivation, NF-κB nuclear translocation was examined by EMSA. A = unstimulated synovial cells; B = synovial cells treated with TNFα for one hour; C = synovial cells treated with TNFα for three hours; D = synovial cells treated with IL1β for one hour; E = synovial cells treated with IL1β for three hours. (B) Suppression of NF-κB nuclear translocation in synovial cells by Z-Leu-Leu-Leu-aldehyde (LLL-CHO). Synovial cells were initially cultured with 10 µM LLL-CHO for six hours, and further incubated with TNFα or IL1β for one hour. After incubation, NF-κB nuclear activity was examined by EMSA. Note that nuclear NF-κB activity in unstimulated, TNFα stimulated, or IL1β stimulated synovial cells was almost suppressed by LLL-CHO. A = unstimulated synovial cells; B = synovial cells stimulated with TNFα ; C = synovial cells stimulated with IL1β ; D = unstimulated synovial cells treated with LLL-CHO; E = TNFα stimulated synovial cells in the presence of LLL-CHO; F = IL1β stimulated synovial cells in the presence of LLL-CHO. Results shown are representative data of five experiments; similar results were obtained from four other experiments.
Figure 3 .
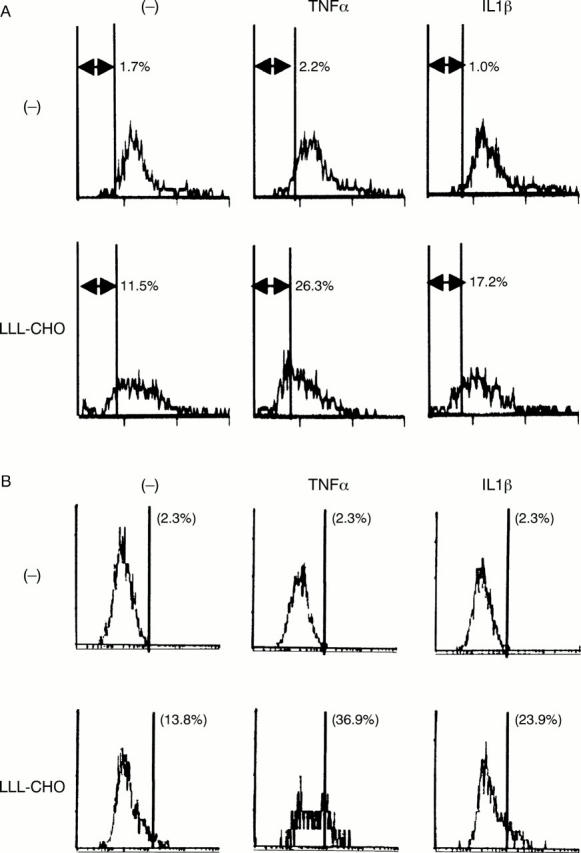
Induction of apoptosis and caspase-3 activation in synovial cells by Z-Leu-Leu-Leu-aldehyde (LLL-CHO). Synovial cells from patients with rheumatoid arthritis (RA) were initially cultured with or without LLL-CHO (10 µM) for six hours, and further incubated in the presence or absence of tumour necrosis factor α (TNFα; 200 IU/ml) or interleukin 1β (IL1β; 20 IU/ml) for an additional 18 hours. After incubation, apoptotic cell death (A) and activation of caspase-3 (B) were examined as described in the text. Note that neither apoptosis nor caspase-3 activation was obvious in unstimulated, TNFα stimulated, or IL1β stimulated synovial cells. Treatment of synovial cells with LLL-CHO induced apoptosis with an increase of intracellular caspase-3 activity, which was higher in TNFα or IL1β stimulated synovial cells. Results are representative data of six experiments; similar results were obtained from five other experiments.
Figure 4 .
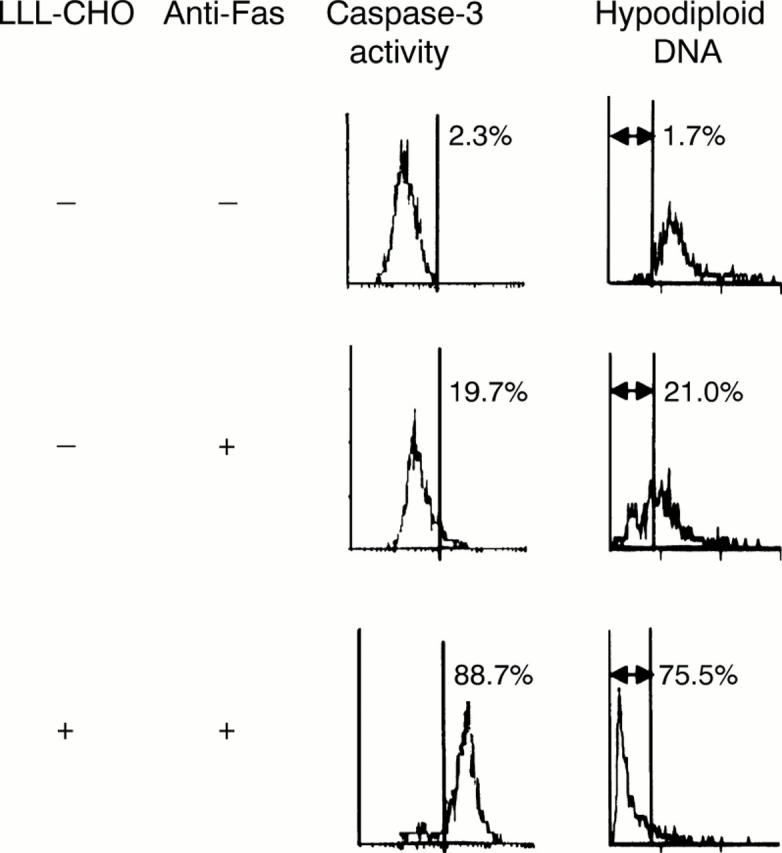
Treatment of synovial cells with Z-Leu-Leu-Leu-aldehyde (LLL-CHO) augments Fas mediated apoptosis of synovial cells. Synovial cells from patients with rheumatoid arthritis (RA) were initially cultured with or without LLL-CHO (10 µM) for six hours, and further incubated in the presence or absence of anti-Fas monoclonal antibody (mAb; 1 µg/ml) for an additional 18 hours. After incubation, apoptotic cell death and activation of caspase-3 were examined as described in the text. LLL-CHO enhanced anti-Fas mAb induced apoptosis with an increase of caspase-3 activity. Results are representative data of six experiments; similar results were obtained from five other experiments.
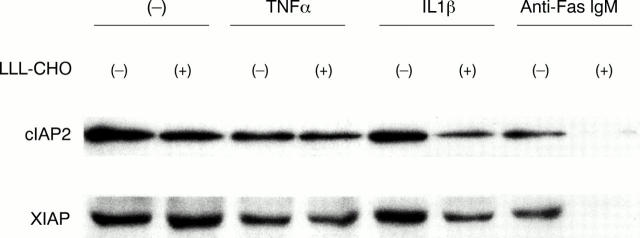
Figure 5 .
cIAP2 and XIAP expression in synovial cells determined by western blot analysis. Synovial cells from patients with rheumatoid arthritis (RA) were initially cultured with or without Z-Leu-Leu-Leu-aldehyde (LLL-CHO; 10 µM) for six hours, and further incubated in the presence or absence of tumour necrosis factor α (TNFα; 200 IU/ml), interleukin 1β (IL1β; 20 IU/ml), or anti-Fas mAb (1 µg/ml) for an additional 18 hours. After incubation, the expression of cIAP2 and XIAP in the cells was examined by western blot analysis as described in the text. Both cIAP2 and XIAP were expressed in unstimulated synovial cells, and the expression was down regulated by TNFα, but not by IL1β. Treatment with LLL-CHO did not affect the expression of cIAP2 and XIAP in unstimulated synovial cells or TNFα stimulated synovial cells. Expression of cIAP2 and XIAP in IL1β stimulated synovial cells was suppressed by LLL-CHO. Treatment of synovial cells with anti-Fas mAb inhibited the expression of cIAP2 and XIAP, which was significantly suppressed by LLL-CHO. Results are representative data of six experiments; similar results were obtained from five other experiments.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arend W. P., Dayer J. M. Cytokines and cytokine inhibitors or antagonists in rheumatoid arthritis. Arthritis Rheum. 1990 Mar;33(3):305–315. doi: 10.1002/art.1780330302. [DOI] [PubMed] [Google Scholar]
- Arnett F. C., Edworthy S. M., Bloch D. A., McShane D. J., Fries J. F., Cooper N. S., Healey L. A., Kaplan S. R., Liang M. H., Luthra H. S. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum. 1988 Mar;31(3):315–324. doi: 10.1002/art.1780310302. [DOI] [PubMed] [Google Scholar]
- Asahara H., Hasumuna T., Kobata T., Yagita H., Okumura K., Inoue H., Gay S., Sumida T., Nishioka K. Expression of Fas antigen and Fas ligand in the rheumatoid synovial tissue. Clin Immunol Immunopathol. 1996 Oct;81(1):27–34. doi: 10.1006/clin.1996.0153. [DOI] [PubMed] [Google Scholar]
- Baeuerle P. A., Baltimore D. NF-kappa B: ten years after. Cell. 1996 Oct 4;87(1):13–20. doi: 10.1016/s0092-8674(00)81318-5. [DOI] [PubMed] [Google Scholar]
- Baeuerle P. A., Henkel T. Function and activation of NF-kappa B in the immune system. Annu Rev Immunol. 1994;12:141–179. doi: 10.1146/annurev.iy.12.040194.001041. [DOI] [PubMed] [Google Scholar]
- Beg A. A., Baltimore D. An essential role for NF-kappaB in preventing TNF-alpha-induced cell death. Science. 1996 Nov 1;274(5288):782–784. doi: 10.1126/science.274.5288.782. [DOI] [PubMed] [Google Scholar]
- Deveraux Q. L., Leo E., Stennicke H. R., Welsh K., Salvesen G. S., Reed J. C. Cleavage of human inhibitor of apoptosis protein XIAP results in fragments with distinct specificities for caspases. EMBO J. 1999 Oct 1;18(19):5242–5251. doi: 10.1093/emboj/18.19.5242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldmann M., Brennan F. M., Maini R. N. Rheumatoid arthritis. Cell. 1996 May 3;85(3):307–310. doi: 10.1016/s0092-8674(00)81109-5. [DOI] [PubMed] [Google Scholar]
- Firestein G. S., Yeo M., Zvaifler N. J. Apoptosis in rheumatoid arthritis synovium. J Clin Invest. 1995 Sep;96(3):1631–1638. doi: 10.1172/JCI118202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujisawa K., Asahara H., Okamoto K., Aono H., Hasunuma T., Kobata T., Iwakura Y., Yonehara S., Sumida T., Nishioka K. Therapeutic effect of the anti-Fas antibody on arthritis in HTLV-1 tax transgenic mice. J Clin Invest. 1996 Jul 15;98(2):271–278. doi: 10.1172/JCI118789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gavrieli Y., Sherman Y., Ben-Sasson S. A. Identification of programmed cell death in situ via specific labeling of nuclear DNA fragmentation. J Cell Biol. 1992 Nov;119(3):493–501. doi: 10.1083/jcb.119.3.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Handel M. L., McMorrow L. B., Gravallese E. M. Nuclear factor-kappa B in rheumatoid synovium. Localization of p50 and p65. Arthritis Rheum. 1995 Dec;38(12):1762–1770. doi: 10.1002/art.1780381209. [DOI] [PubMed] [Google Scholar]
- Joosten L. A., Helsen M. M., van de Loo F. A., van den Berg W. B. Anticytokine treatment of established type II collagen-induced arthritis in DBA/1 mice. A comparative study using anti-TNF alpha, anti-IL-1 alpha/beta, and IL-1Ra. Arthritis Rheum. 1996 May;39(5):797–809. doi: 10.1002/art.1780390513. [DOI] [PubMed] [Google Scholar]
- Kawakami A., Eguchi K., Matsuoka N., Tsuboi M., Kawabe Y., Aoyagi T., Nagataki S. Inhibition of Fas antigen-mediated apoptosis of rheumatoid synovial cells in vitro by transforming growth factor beta 1. Arthritis Rheum. 1996 Aug;39(8):1267–1276. doi: 10.1002/art.1780390802. [DOI] [PubMed] [Google Scholar]
- Kawakami A., Nakashima T., Sakai H., Hida A., Urayama S., Yamasaki S., Nakamura H., Ida H., Ichinose Y., Aoyagi T. Regulation of synovial cell apoptosis by proteasome inhibitor. Arthritis Rheum. 1999 Nov;42(11):2440–2448. doi: 10.1002/1529-0131(199911)42:11<2440::AID-ANR23>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- Kobayashi T., Okamoto K., Kobata T., Hasunuma T., Sumida T., Nishioka K. Tumor necrosis factor alpha regulation of the FAS-mediated apoptosis-signaling pathway in synovial cells. Arthritis Rheum. 1999 Mar;42(3):519–526. doi: 10.1002/1529-0131(199904)42:3<519::AID-ANR17>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- Koh H., Lee K. H., Kim D., Kim S., Kim J. W., Chung J. Inhibition of Akt and its anti-apoptotic activities by tumor necrosis factor-induced protein kinase C-related kinase 2 (PRK2) cleavage. J Biol Chem. 2000 Nov 3;275(44):34451–34458. doi: 10.1074/jbc.M001753200. [DOI] [PubMed] [Google Scholar]
- Liu Z. G., Hsu H., Goeddel D. V., Karin M. Dissection of TNF receptor 1 effector functions: JNK activation is not linked to apoptosis while NF-kappaB activation prevents cell death. Cell. 1996 Nov 1;87(3):565–576. doi: 10.1016/s0092-8674(00)81375-6. [DOI] [PubMed] [Google Scholar]
- Marok R., Winyard P. G., Coumbe A., Kus M. L., Gaffney K., Blades S., Mapp P. I., Morris C. J., Blake D. R., Kaltschmidt C. Activation of the transcription factor nuclear factor-kappaB in human inflamed synovial tissue. Arthritis Rheum. 1996 Apr;39(4):583–591. doi: 10.1002/art.1780390407. [DOI] [PubMed] [Google Scholar]
- Miagkov A. V., Kovalenko D. V., Brown C. E., Didsbury J. R., Cogswell J. P., Stimpson S. A., Baldwin A. S., Makarov S. S. NF-kappaB activation provides the potential link between inflammation and hyperplasia in the arthritic joint. Proc Natl Acad Sci U S A. 1998 Nov 10;95(23):13859–13864. doi: 10.1073/pnas.95.23.13859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakajima T., Aono H., Hasunuma T., Yamamoto K., Shirai T., Hirohata K., Nishioka K. Apoptosis and functional Fas antigen in rheumatoid arthritis synoviocytes. Arthritis Rheum. 1995 Apr;38(4):485–491. doi: 10.1002/art.1780380405. [DOI] [PubMed] [Google Scholar]
- Ninomiya-Tsuji J., Kishimoto K., Hiyama A., Inoue J., Cao Z., Matsumoto K. The kinase TAK1 can activate the NIK-I kappaB as well as the MAP kinase cascade in the IL-1 signalling pathway. Nature. 1999 Mar 18;398(6724):252–256. doi: 10.1038/18465. [DOI] [PubMed] [Google Scholar]
- Palombella V. J., Rando O. J., Goldberg A. L., Maniatis T. The ubiquitin-proteasome pathway is required for processing the NF-kappa B1 precursor protein and the activation of NF-kappa B. Cell. 1994 Sep 9;78(5):773–785. doi: 10.1016/s0092-8674(94)90482-0. [DOI] [PubMed] [Google Scholar]
- Pousset F., Dantzer R., Kelley K. W., Parnet P. Interleukin-1 signaling in mouse astrocytes involves Akt: a study with interleukin-4 and IL-10. Eur Cytokine Netw. 2000 Sep;11(3):427–434. [PubMed] [Google Scholar]
- Romashkova J. A., Makarov S. S. NF-kappaB is a target of AKT in anti-apoptotic PDGF signalling. Nature. 1999 Sep 2;401(6748):86–90. doi: 10.1038/43474. [DOI] [PubMed] [Google Scholar]
- Roy N., Deveraux Q. L., Takahashi R., Salvesen G. S., Reed J. C. The c-IAP-1 and c-IAP-2 proteins are direct inhibitors of specific caspases. EMBO J. 1997 Dec 1;16(23):6914–6925. doi: 10.1093/emboj/16.23.6914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stehlik C., de Martin R., Kumabashiri I., Schmid J. A., Binder B. R., Lipp J. Nuclear factor (NF)-kappaB-regulated X-chromosome-linked iap gene expression protects endothelial cells from tumor necrosis factor alpha-induced apoptosis. J Exp Med. 1998 Jul 6;188(1):211–216. doi: 10.1084/jem.188.1.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi R., Deveraux Q., Tamm I., Welsh K., Assa-Munt N., Salvesen G. S., Reed J. C. A single BIR domain of XIAP sufficient for inhibiting caspases. J Biol Chem. 1998 Apr 3;273(14):7787–7790. doi: 10.1074/jbc.273.14.7787. [DOI] [PubMed] [Google Scholar]
- Tomita T., Takeuchi E., Tomita N., Morishita R., Kaneko M., Yamamoto K., Nakase T., Seki H., Kato K., Kaneda Y. Suppressed severity of collagen-induced arthritis by in vivo transfection of nuclear factor kappaB decoy oligodeoxynucleotides as a gene therapy. Arthritis Rheum. 1999 Dec;42(12):2532–2542. doi: 10.1002/1529-0131(199912)42:12<2532::AID-ANR5>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- Vaishnaw A. K., McNally J. D., Elkon K. B. Apoptosis in the rheumatic diseases. Arthritis Rheum. 1997 Nov;40(11):1917–1927. doi: 10.1002/art.1780401102. [DOI] [PubMed] [Google Scholar]
- Van Antwerp D. J., Martin S. J., Kafri T., Green D. R., Verma I. M. Suppression of TNF-alpha-induced apoptosis by NF-kappaB. Science. 1996 Nov 1;274(5288):787–789. doi: 10.1126/science.274.5288.787. [DOI] [PubMed] [Google Scholar]
- Vucic D., Kaiser W. J., Harvey A. J., Miller L. K. Inhibition of reaper-induced apoptosis by interaction with inhibitor of apoptosis proteins (IAPs). Proc Natl Acad Sci U S A. 1997 Sep 16;94(19):10183–10188. doi: 10.1073/pnas.94.19.10183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C. Y., Mayo M. W., Korneluk R. G., Goeddel D. V., Baldwin A. S., Jr NF-kappaB antiapoptosis: induction of TRAF1 and TRAF2 and c-IAP1 and c-IAP2 to suppress caspase-8 activation. Science. 1998 Sep 11;281(5383):1680–1683. doi: 10.1126/science.281.5383.1680. [DOI] [PubMed] [Google Scholar]