Abstract
OBJECTIVES—To study the short term effects of a single dose of D2E7, a fully human anti-tumour necrosis factor (TNFα) monoclonal antibody (mAb), on the local and systemic homeostasis of interleukin 1β (IL1β) and TNFα in patients with rheumatoid arthritis (RA). METHODS—All patients with RA enrolled in a phase I, single dose, placebo controlled study with D2E7 in our centre were studied. Systemic cytokine levels, acute phase reactants, and leucocyte counts were studied at days 0, 1, and 14 after the first administration of anti-TNF mAb (n=39) or placebo (n=11). The cellularity and the expression of IL1 and TNFα in synovial tissue were studied in knee biopsy specimens obtained at baseline and at day 14 in 25 consenting patients. RESULTS—A single dose of anti-TNF mAb induced a rapid clinical improvement, a decrease in acute phase reaction, and increased lymphocyte counts in patients with active RA. The protein levels of IL1β in the circulation were low and remained unchanged, but the systemic levels of IL1β mRNA (p=0.002) and the concentrations of IL1 receptor antagonist (IL1ra) and IL6 (p=0.0001) had already dropped within 24 hours and this persisted up to day 14. Systemic levels of TNFα mRNA were low and remained unchanged, though total TNFα (free and bound) in the circulation increased after D2E7, probably reflecting the presence of TNF-antiTNF mAb complexes (p<0.005, at days 1 and 14). Both TNF receptors dropped below baseline levels at day 14 (p<0.005). Despite clinical improvement of arthritis, no consistent immunohistological changes were seen two weeks after anti-TNF administration. Endothelial staining for IL1β tended to decrease in treated patients (p=0.06) but not in responders. The staining for IL1β and TNFα in sublining layers and vessels was mutually correlated (rs=0.47 and 0.58 respectively, p<0.0005) and the microscopic scores for inflammation correlated with sublining TNFα and IL1β scores (rs=0.65 and 0.54 respectively, p<0.0001), though none of these showed significant changes during the study. CONCLUSIONS—Blocking TNFα in RA results in down regulation of IL1β mRNA at the systemic level and in reduction of the endogenous antagonists for IL1 and TNF and of other cytokines related to the acute phase response, such as IL6, within days. At the synovial level, anti-TNF treatment does not modulate IL1β and TNFα in the short term. The synovial expression of these cytokines does not reflect clinical response to TNF neutralisation.
Full Text
The Full Text of this article is available as a PDF (307.4 KB).
Figure 1 .

(A) Disease activity score (DAS), (B) erythrocyte sedimentation rate (ESR), and (C) C reactive protein (CRP) in patients receiving a single dose of placebo or 0.5 mg/kg or 1-10 mg/kg anti-tumour necrosis factor monoclonal antibody. Boxes represent the 25th, 50th, and 75th centiles; vertical lines indicate the 5th and 95th centiles. Measurements on days 0, 1, and 14 after infusion (x axis). Comparisons versus baseline shown as *p<0.05, **p<0.005, and ***p<0.0005.
Figure 2 .

Circulating concentrations of (A) interleukin 1 receptor antagonist (IL1ra), (B) IL6, (C) 55th centile (p55), and (D) 75th centile (p75) soluble tumour necrosis factor receptors (sTNFR). (E) IL1β and (F) TNFα mRNA in whole blood normalised for the presence of β2 microglobulin (β2M). Boxes represent 25th, 50th, and 75th centiles; vertical lines indicate the 5th and 95th centiles. Measurements on days 0, 1, and 14 after infusion (x axis). Comparisons versus baseline shown as *p<0.5, **p<0.005, and ***p<0.0005.
Figure 3 .
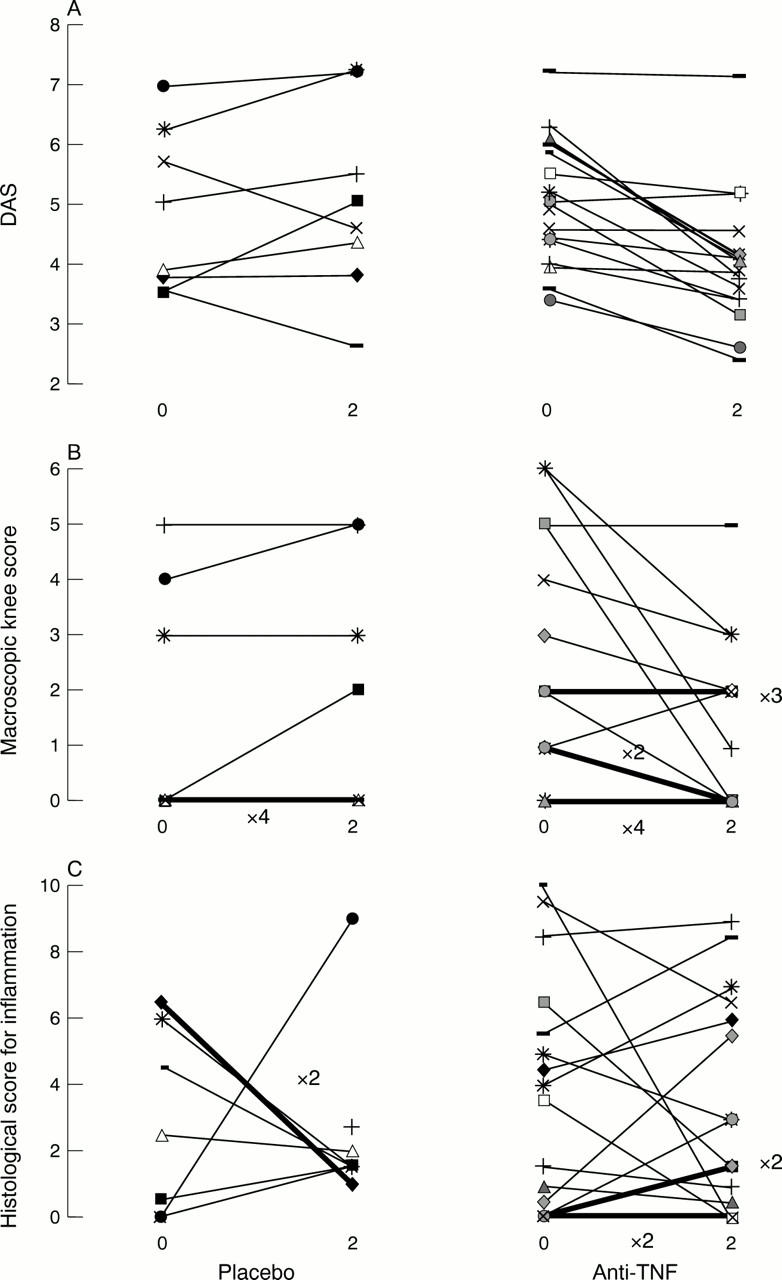
(A) Disease activity score (DAS), (B) pain and swelling scores, and (C) histological scores for inflammation in 25 patients undergoing serial biopsies. Time in weeks and placebo (n=8) or anti-tumour necrosis factor (anti-TNF) treatment (n=17) shown in the x axis. Scores for more than one patient (× exact number) shown as bold lines.
Figure 4 .
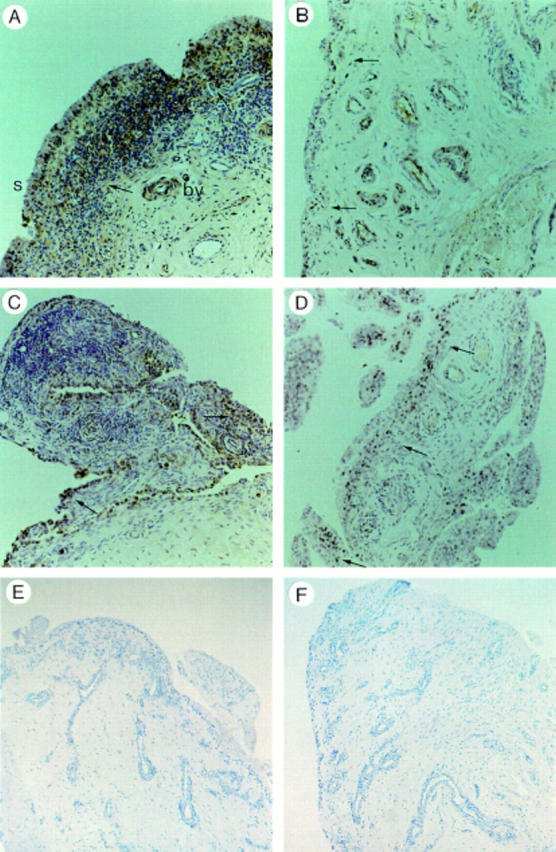
Immunohistochemical staining in the same patient at baseline ((A) tumour necrosis factor α (TNFα), (B) interleukin 1β (IL1β)) and 14 days after ((C) TNFα, (D) IL1β) the first dose of anti-TNFα. Note the unchanged expression of cytokines in the synovial lining (s, arrows), sublining, and blood vessels (bv). No IL1β or TNFα staining was seen in controls with (E) an irrelevant primary antibody and (F) when the secondary antibody was omitted. Original magnification ×200.
Figure 5 .
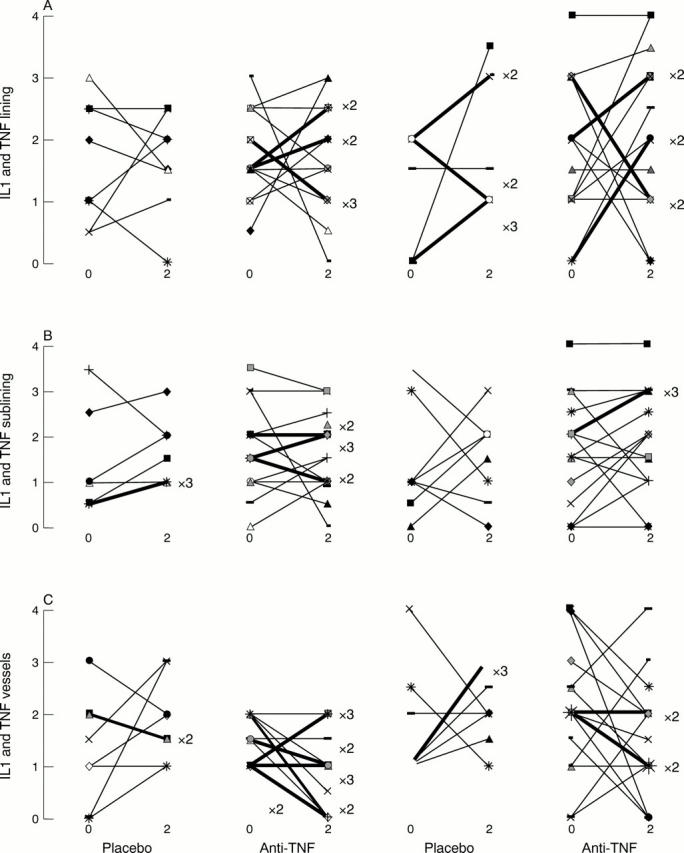
Semiquantitative scores for interleukin 1β (IL1β; left columns) and tumour necrosis factor α (TNFα; right columns). Staining in (A) lining, (B) sublining, and (C) vessels in biopsy specimens taken at baseline and at day 14 after administration of placebo (n=8) or anti-TNF treatment (n=17). Scores for more than one patient (× exact number) shown as bold lines.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnett F. C., Edworthy S. M., Bloch D. A., McShane D. J., Fries J. F., Cooper N. S., Healey L. A., Kaplan S. R., Liang M. H., Luthra H. S. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum. 1988 Mar;31(3):315–324. doi: 10.1002/art.1780310302. [DOI] [PubMed] [Google Scholar]
- Barrera P., Blom A., van Lent P. L., van Bloois L., Beijnen J. H., van Rooijen N., de Waal Malefijt M. C., van de Putte L. B., Storm G., van den Berg W. B. Synovial macrophage depletion with clodronate-containing liposomes in rheumatoid arthritis. Arthritis Rheum. 2000 Sep;43(9):1951–1959. doi: 10.1002/1529-0131(200009)43:9<1951::AID-ANR5>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- Barrera P., Boerbooms A. M., Janssen E. M., Sauerwein R. W., Gallati H., Mulder J., de Boo T., Demacker P. N., van de Putte L. B., van der Meer J. W. Circulating soluble tumor necrosis factor receptors, interleukin-2 receptors, tumor necrosis factor alpha, and interleukin-6 levels in rheumatoid arthritis. Longitudinal evaluation during methotrexate and azathioprine therapy. Arthritis Rheum. 1993 Aug;36(8):1070–1079. doi: 10.1002/art.1780360807. [DOI] [PubMed] [Google Scholar]
- Bleeker M. W., Netea M. G., Kullberg B. J., Van der Ven-Jongekrijg J., Van der Meer J. W. The effects of dexamethasone and chlorpromazine on tumour necrosis factor-alpha, interleukin-1 beta, interleukin-1 receptor antagonist and interleukin-10 in human volunteers. Immunology. 1997 Aug;91(4):548–552. doi: 10.1046/j.1365-2567.1997.00285.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brennan F. M., Chantry D., Jackson A., Maini R., Feldmann M. Inhibitory effect of TNF alpha antibodies on synovial cell interleukin-1 production in rheumatoid arthritis. Lancet. 1989 Jul 29;2(8657):244–247. doi: 10.1016/s0140-6736(89)90430-3. [DOI] [PubMed] [Google Scholar]
- Bresnihan B. Treatment of rheumatoid arthritis with interleukin 1 receptor antagonist. Ann Rheum Dis. 1999 Nov;58 (Suppl 1):I96–I98. doi: 10.1136/ard.58.2008.i96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler D. M., Maini R. N., Feldmann M., Brennan F. M. Modulation of proinflammatory cytokine release in rheumatoid synovial membrane cell cultures. Comparison of monoclonal anti TNF-alpha antibody with the interleukin-1 receptor antagonist. Eur Cytokine Netw. 1995 Jul-Dec;6(4):225–230. [PubMed] [Google Scholar]
- Davis D., Charles P. J., Potter A., Feldmann M., Maini R. N., Elliott M. J. Anaemia of chronic disease in rheumatoid arthritis: in vivo effects of tumour necrosis factor alpha blockade. Br J Rheumatol. 1997 Sep;36(9):950–956. doi: 10.1093/rheumatology/36.9.950. [DOI] [PubMed] [Google Scholar]
- Dinarello C. A. Biologic basis for interleukin-1 in disease. Blood. 1996 Mar 15;87(6):2095–2147. [PubMed] [Google Scholar]
- Elliott M. J., Maini R. N., Feldmann M., Kalden J. R., Antoni C., Smolen J. S., Leeb B., Breedveld F. C., Macfarlane J. D., Bijl H. Randomised double-blind comparison of chimeric monoclonal antibody to tumour necrosis factor alpha (cA2) versus placebo in rheumatoid arthritis. Lancet. 1994 Oct 22;344(8930):1105–1110. doi: 10.1016/s0140-6736(94)90628-9. [DOI] [PubMed] [Google Scholar]
- Feldmann M., Brennan F., Paleolog E., Taylor P., Maini R. N. Anti-tumor necrosis factor alpha therapy of rheumatoid arthritis. Mechanism of action. Eur Cytokine Netw. 1997 Sep;8(3):297–300. [PubMed] [Google Scholar]
- Jouvenne P., Vannier E., Dinarello C. A., Miossec P. Elevated levels of soluble interleukin-1 receptor type II and interleukin-1 receptor antagonist in patients with chronic arthritis: correlations with markers of inflammation and joint destruction. Arthritis Rheum. 1998 Jun;41(6):1083–1089. doi: 10.1002/1529-0131(199806)41:6<1083::AID-ART15>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- Kempeni J. Preliminary results of early clinical trials with the fully human anti-TNFalpha monoclonal antibody D2E7. Ann Rheum Dis. 1999 Nov;58 (Suppl 1):I70–I72. doi: 10.1136/ard.58.2008.i70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuiper S., Joosten L. A., Bendele A. M., Edwards C. K., 3rd, Arntz O. J., Helsen M. M., Van de Loo F. A., Van den Berg W. B. Different roles of tumour necrosis factor alpha and interleukin 1 in murine streptococcal cell wall arthritis. Cytokine. 1998 Sep;10(9):690–702. doi: 10.1006/cyto.1998.0372. [DOI] [PubMed] [Google Scholar]
- Lorenz H. M., Antoni C., Valerius T., Repp R., Grünke M., Schwerdtner N., Nüsslein H., Woody J., Kalden J. R., Manger B. In vivo blockade of TNF-alpha by intravenous infusion of a chimeric monoclonal TNF-alpha antibody in patients with rheumatoid arthritis. Short term cellular and molecular effects. J Immunol. 1996 Feb 15;156(4):1646–1653. [PubMed] [Google Scholar]
- Maini R. N., Breedveld F. C., Kalden J. R., Smolen J. S., Davis D., Macfarlane J. D., Antoni C., Leeb B., Elliott M. J., Woody J. N. Therapeutic efficacy of multiple intravenous infusions of anti-tumor necrosis factor alpha monoclonal antibody combined with low-dose weekly methotrexate in rheumatoid arthritis. Arthritis Rheum. 1998 Sep;41(9):1552–1563. doi: 10.1002/1529-0131(199809)41:9<1552::AID-ART5>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- Moreland L. W., Schiff M. H., Baumgartner S. W., Tindall E. A., Fleischmann R. M., Bulpitt K. J., Weaver A. L., Keystone E. C., Furst D. E., Mease P. J. Etanercept therapy in rheumatoid arthritis. A randomized, controlled trial. Ann Intern Med. 1999 Mar 16;130(6):478–486. doi: 10.7326/0003-4819-130-6-199903160-00004. [DOI] [PubMed] [Google Scholar]
- Netea M. G., Drenth J. P., De Bont N., Hijmans A., Keuter M., Dharmana E., Demacker P. N., van der Meer J. W. A semi-quantitative reverse transcriptase polymerase chain reaction method for measurement of MRNA for TNF-alpha and IL-1 beta in whole blood cultures: its application in typhoid fever and exentric exercise. Cytokine. 1996 Sep;8(9):739–744. doi: 10.1006/cyto.1996.0098. [DOI] [PubMed] [Google Scholar]
- Paleolog E. M., Hunt M., Elliott M. J., Feldmann M., Maini R. N., Woody J. N. Deactivation of vascular endothelium by monoclonal anti-tumor necrosis factor alpha antibody in rheumatoid arthritis. Arthritis Rheum. 1996 Jul;39(7):1082–1091. doi: 10.1002/art.1780390703. [DOI] [PubMed] [Google Scholar]
- Paleolog E. M., Young S., Stark A. C., McCloskey R. V., Feldmann M., Maini R. N. Modulation of angiogenic vascular endothelial growth factor by tumor necrosis factor alpha and interleukin-1 in rheumatoid arthritis. Arthritis Rheum. 1998 Jul;41(7):1258–1265. doi: 10.1002/1529-0131(199807)41:7<1258::AID-ART17>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- Perkins D. J., St Clair E. W., Misukonis M. A., Weinberg J. B. Reduction of NOS2 overexpression in rheumatoid arthritis patients treated with anti-tumor necrosis factor alpha monoclonal antibody (cA2). Arthritis Rheum. 1998 Dec;41(12):2205–2210. doi: 10.1002/1529-0131(199812)41:12<2205::AID-ART16>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- Present D. H., Rutgeerts P., Targan S., Hanauer S. B., Mayer L., van Hogezand R. A., Podolsky D. K., Sands B. E., Braakman T., DeWoody K. L. Infliximab for the treatment of fistulas in patients with Crohn's disease. N Engl J Med. 1999 May 6;340(18):1398–1405. doi: 10.1056/NEJM199905063401804. [DOI] [PubMed] [Google Scholar]
- Rankin E. C., Choy E. H., Kassimos D., Kingsley G. H., Sopwith A. M., Isenberg D. A., Panayi G. S. The therapeutic effects of an engineered human anti-tumour necrosis factor alpha antibody (CDP571) in rheumatoid arthritis. Br J Rheumatol. 1995 Apr;34(4):334–342. doi: 10.1093/rheumatology/34.4.334. [DOI] [PubMed] [Google Scholar]
- Rodenburg R. J., van Den Hoogen F. H., Barrera P., van Venrooij W. J., van De Putte L. B. Superinduction of interleukin 8 mRNA in activated monocyte derived macrophages from rheumatoid arthritis patients. Ann Rheum Dis. 1999 Oct;58(10):648–652. doi: 10.1136/ard.58.10.648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulze-Koops H., Davis L. S., Kavanaugh A. F., Lipsky P. E. Elevated cytokine messenger RNA levels in the peripheral blood of patients with rheumatoid arthritis suggest different degrees of myeloid cell activation. Arthritis Rheum. 1997 Apr;40(4):639–647. doi: 10.1002/art.1780400408. [DOI] [PubMed] [Google Scholar]
- Stack W. A., Mann S. D., Roy A. J., Heath P., Sopwith M., Freeman J., Holmes G., Long R., Forbes A., Kamm M. A. Randomised controlled trial of CDP571 antibody to tumour necrosis factor-alpha in Crohn's disease. Lancet. 1997 Feb 22;349(9051):521–524. doi: 10.1016/s0140-6736(97)80083-9. [DOI] [PubMed] [Google Scholar]
- Tak P. P., Taylor P. C., Breedveld F. C., Smeets T. J., Daha M. R., Kluin P. M., Meinders A. E., Maini R. N. Decrease in cellularity and expression of adhesion molecules by anti-tumor necrosis factor alpha monoclonal antibody treatment in patients with rheumatoid arthritis. Arthritis Rheum. 1996 Jul;39(7):1077–1081. doi: 10.1002/art.1780390702. [DOI] [PubMed] [Google Scholar]
- Ulfgren A. K., Andersson U., Engström M., Klareskog L., Maini R. N., Taylor P. C. Systemic anti-tumor necrosis factor alpha therapy in rheumatoid arthritis down-regulates synovial tumor necrosis factor alpha synthesis. Arthritis Rheum. 2000 Nov;43(11):2391–2396. doi: 10.1002/1529-0131(200011)43:11<2391::AID-ANR3>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- Youssef P. P., Haynes D. R., Triantafillou S., Parker A., Gamble J. R., Roberts-Thomson P. J., Ahern M. J., Smith M. D. Effects of pulse methylprednisolone on inflammatory mediators in peripheral blood, synovial fluid, and synovial membrane in rheumatoid arthritis. Arthritis Rheum. 1997 Aug;40(8):1400–1408. doi: 10.1002/art.1780400807. [DOI] [PubMed] [Google Scholar]
- van Deuren M., Netea M. G., Hijmans A., Demacker P. N., Neeleman C., Sauerwein R. W., Bartelink A. K., van der Meer J. W. Posttranscriptional down-regulation of tumor necrosis factor-alpha and interleukin-1beta production in acute meningococcal infections. J Infect Dis. 1998 May;177(5):1401–1405. doi: 10.1086/517824. [DOI] [PubMed] [Google Scholar]
- van Gestel A. M., Prevoo M. L., van 't Hof M. A., van Rijswijk M. H., van de Putte L. B., van Riel P. L. Development and validation of the European League Against Rheumatism response criteria for rheumatoid arthritis. Comparison with the preliminary American College of Rheumatology and the World Health Organization/International League Against Rheumatism Criteria. Arthritis Rheum. 1996 Jan;39(1):34–40. doi: 10.1002/art.1780390105. [DOI] [PubMed] [Google Scholar]
- van de Loo F. A., Joosten L. A., van Lent P. L., Arntz O. J., van den Berg W. B. Role of interleukin-1, tumor necrosis factor alpha, and interleukin-6 in cartilage proteoglycan metabolism and destruction. Effect of in situ blocking in murine antigen- and zymosan-induced arthritis. Arthritis Rheum. 1995 Feb;38(2):164–172. doi: 10.1002/art.1780380204. [DOI] [PubMed] [Google Scholar]
- van den Berg W. B., Joosten L. A., Kollias G., van De Loo F. A. Role of tumour necrosis factor alpha in experimental arthritis: separate activity of interleukin 1beta in chronicity and cartilage destruction. Ann Rheum Dis. 1999 Nov;58 (Suppl 1):I40–I48. doi: 10.1136/ard.58.2008.i40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Heijde D. M., van 't Hof M. A., van Riel P. L., Theunisse L. A., Lubberts E. W., van Leeuwen M. A., van Rijswijk M. H., van de Putte L. B. Judging disease activity in clinical practice in rheumatoid arthritis: first step in the development of a disease activity score. Ann Rheum Dis. 1990 Nov;49(11):916–920. doi: 10.1136/ard.49.11.916. [DOI] [PMC free article] [PubMed] [Google Scholar]


