Abstract
BACKGROUND—Excess tissue matrix accumulates in systemic sclerosis (SSc), accounting for both visceral and dermal fibrosis. It is suggested that decreased serum levels of matrix metalloproteinases (MMPs) or increased levels of tissue inhibitors of matrix metalloproteinases (TIMPs) may account for this matrix accumulation. OBJECTIVE—To measure serum levels of tissue inhibitors of metalloproteinases, TIMP-1, TIMP-2, and collagenase-1 (MMP-1), in patients with diffuse cutaneous systemic sclerosis (dcSSc), limited cutaneous systemic sclerosis (lcSSc), primary Raynaud's phenomenon (RP), and in normal controls. METHODS—Serum samples from patients with dcSSc (n=83), lcSSc (n=87), RP (n=80), and normal controls (n=98) were analysed using enzyme linked immunosorbent assays (ELISAs) for total TIMP-1, TIMP-2, and MMP-1. Results from each assay were analysed by the Kruskal-Wallis test. Dunn's multiple comparison post-test was then applied between groups. RESULTS—TIMP-1 levels were significantly raised in dcSSc and lcSSc groups compared with the RP group and normal controls (p<0.01 to p<0.001). In the dcSSc group, TIMP-1 levels were significantly higher in early disease (<2 years) than in late stage disease (>4 years) (p<0.05). This was not found for the lcSSc group. Serum TIMP-2 and MMP-1 levels in dcSSc and lcSSc did not differ significantly from those in normal controls. Increased levels of TIMPs were not convincingly associated with organ disease. No assay result correlated with autoantibody status (anti-topoisomerase 1 (anti-Scl-70), anticentromere antibody, or anti-RNA polymerase). No significant differences in serum TIMP-1, TIMP-2, or MMP-1 levels were shown in the RP group compared with normal controls. CONCLUSIONS—Raised TIMP-1 levels in the SSc groups support the hypothesis that matrix accumulation occurs in SSc at least in part owing to decreased degradation. Moreover, the variation in TIMP-1 levels between the early and late disease stages of dcSSc seems to reflect the early progressive course of dermal fibrosis seen clinically. The expected reduction in serum MMP-1 levels in the SSc groups was not found. This suggests that tissue matrix accumulation is due to increased inhibitors rather than to decreased MMPs.
Full Text
The Full Text of this article is available as a PDF (139.5 KB).
Figure 1 .
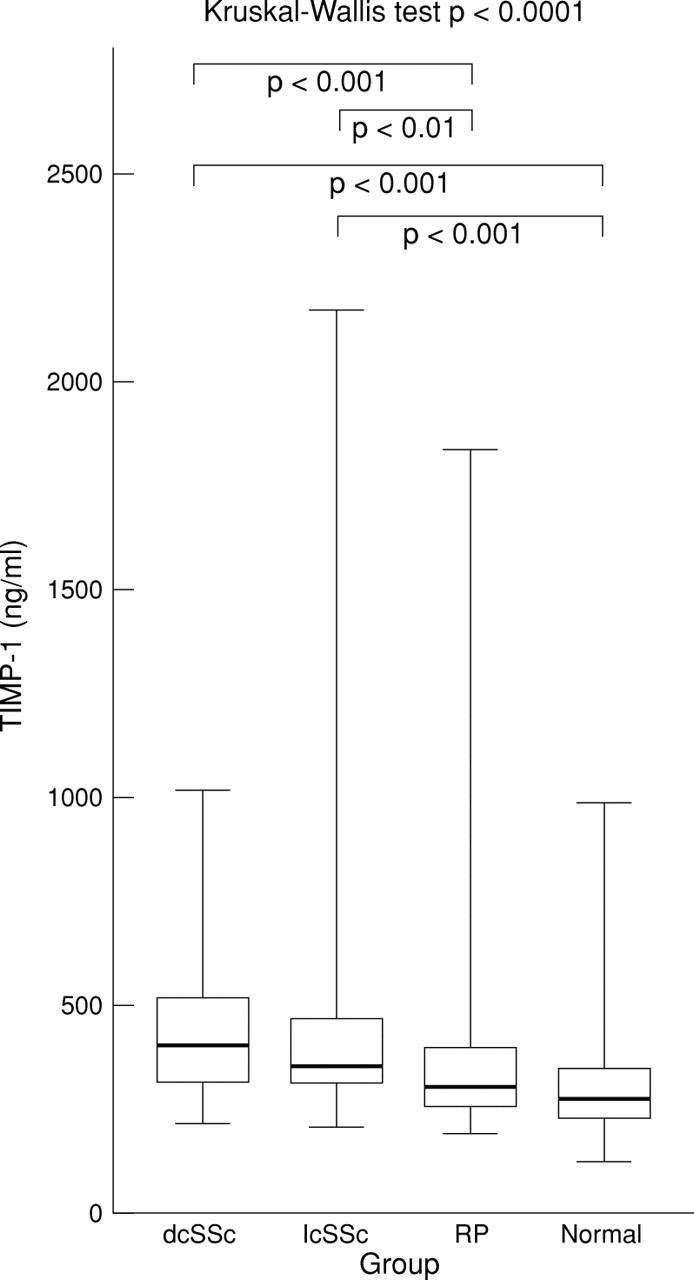
Serum TIMP-1 level in diffuse cutaneous systemic sclerosis (dcSSc), limited cutaneous systemic sclerosis (lcSSc), Raynaud's phenomenon (RP), and normal groups. Box plots with upper and lower bars showing the data range, and upper, middle, and lower lines in the box showing 75th, 50th (median), and 25th centiles respectively. Kruskal-Wallis test across all four groups p<0.0001. Only significant Dunn's multiple comparison post-test p values between groups shown.
Figure 2 .
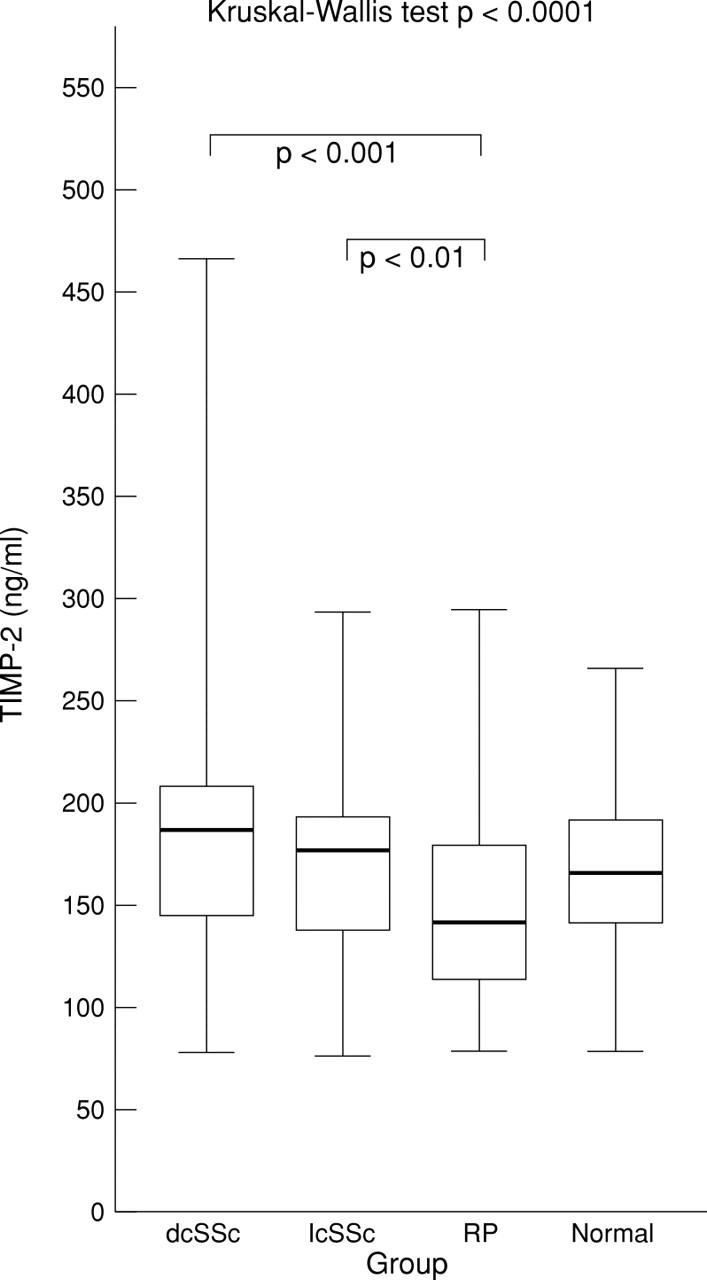
Serum TIMP-2 level in diffuse cutaneous systemic sclerosis (dcSSc), limited cutaneous systemic sclerosis (lcSSc), Raynaud's phenomenon (RP), and normal groups. Box plots with upper and lower bars showing the data range, and upper, middle, and lower lines in the box showing 75th, 50th (median), and 25th centiles respectively. Kruskal-Wallis test across all four groups p<0.0001. Only significant Dunn's multiple comparison post-test p values between groups shown.
Figure 3 .
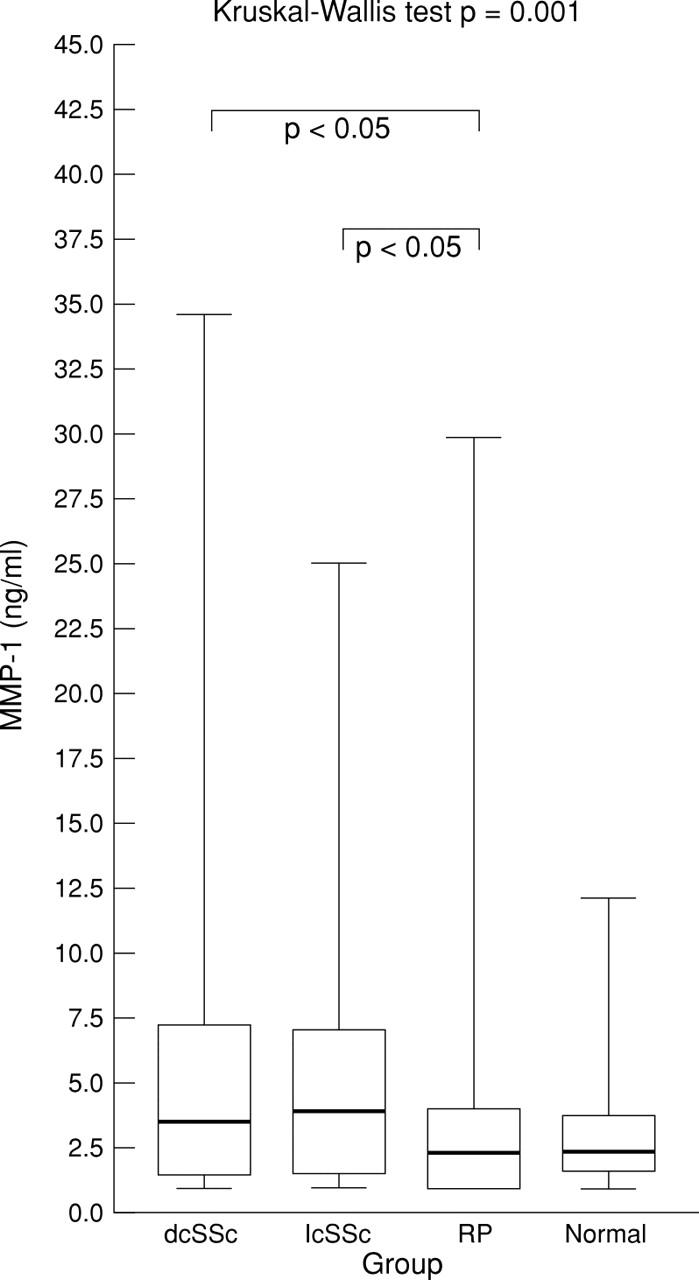
Serum MMP-1 level in diffuse cutaneous systemic sclerosis (dcSSc), limited cutaneous systemic sclerosis (lcSSc), Raynaud's phenomenon (RP), and normal groups. Box plots with upper and lower bars showing the data range, and upper, middle, and lower lines in the box showing 75th, 50th (median), and 25th centiles respectively. Kruskal-Wallis test across all four groups p=0.001. Only significant Dunn's multiple comparison post-test p values between groups shown.
Figure 4 .
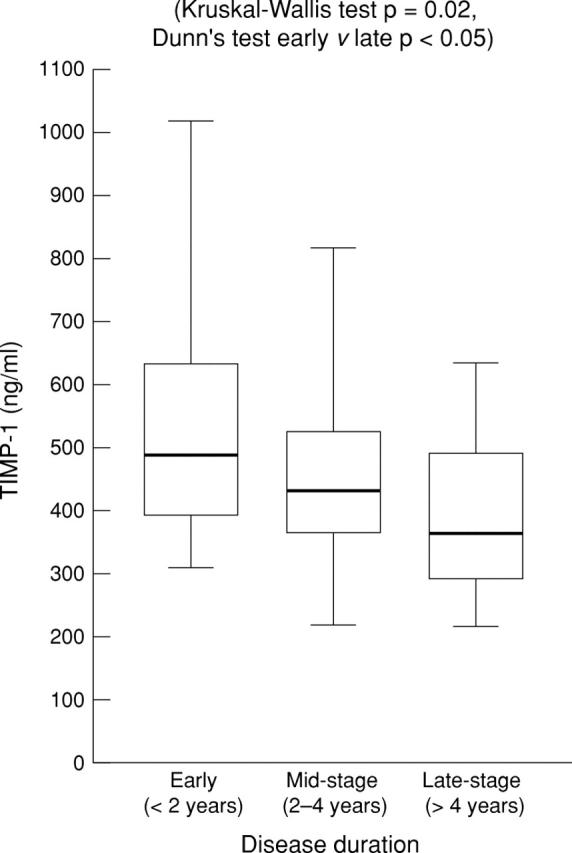
Serum TIMP-1 level in diffuse cutaneous systemic sclerosis (dcSSc)—reduction in level at different disease stages. Box plots with upper and lower bars showing the data range, and upper, middle, and lower lines in the box showing 75th, 50th (median), and 25th centiles respectively. Kruskal-Wallis test across all three groups p=0.02. Dunn's multiple comparison post-test between early and late stages p<0.05.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bou-Gharios G., Osman J., Black C., Olsen I. Excess matrix accumulation in scleroderma is caused partly by differential regulation of stromelysin and TIMP-1 synthesis. Clin Chim Acta. 1994 Nov;231(1):69–78. doi: 10.1016/0009-8981(94)90255-0. [DOI] [PubMed] [Google Scholar]
- Clark I. M., Powell L. K., Wright J. K., Cawston T. E., Hazleman B. L. Monoclonal antibodies against human fibroblast collagenase and the design of an enzyme-linked immunosorbent assay to measure total collagenase. Matrix. 1992 Dec;12(6):475–480. doi: 10.1016/s0934-8832(11)80092-2. [DOI] [PubMed] [Google Scholar]
- Kikuchi K., Kadono T., Furue M., Tamaki K. Tissue inhibitor of metalloproteinase 1 (TIMP-1) may be an autocrine growth factor in scleroderma fibroblasts. J Invest Dermatol. 1997 Mar;108(3):281–284. doi: 10.1111/1523-1747.ep12286457. [DOI] [PubMed] [Google Scholar]
- Kikuchi K., Kubo M., Sato S., Fujimoto M., Tamaki K. Serum tissue inhibitor of metalloproteinases in patients with systemic sclerosis. J Am Acad Dermatol. 1995 Dec;33(6):973–978. doi: 10.1016/0190-9622(95)90289-9. [DOI] [PubMed] [Google Scholar]
- Kirk T. Z., Mark M. E., Chua C. C., Chua B. H., Mayes M. D. Myofibroblasts from scleroderma skin synthesize elevated levels of collagen and tissue inhibitor of metalloproteinase (TIMP-1) with two forms of TIMP-1. J Biol Chem. 1995 Feb 17;270(7):3423–3428. doi: 10.1074/jbc.270.7.3423. [DOI] [PubMed] [Google Scholar]
- Kuroda K., Shinkai H. Gene expression of types I and III collagen, decorin, matrix metalloproteinases and tissue inhibitors of metalloproteinases in skin fibroblasts from patients with systemic sclerosis. Arch Dermatol Res. 1997 Sep;289(10):567–572. doi: 10.1007/s004030050241. [DOI] [PubMed] [Google Scholar]
- LeRoy E. C., Black C., Fleischmajer R., Jablonska S., Krieg T., Medsger T. A., Jr, Rowell N., Wollheim F. Scleroderma (systemic sclerosis): classification, subsets and pathogenesis. J Rheumatol. 1988 Feb;15(2):202–205. [PubMed] [Google Scholar]
- Manicourt D. H., Fujimoto N., Obata K., Thonar E. J. Serum levels of collagenase, stromelysin-1, and TIMP-1. Age- and sex-related differences in normal subjects and relationship to the extent of joint involvement and serum levels of antigenic keratan sulfate in patients with osteoarthritis. Arthritis Rheum. 1994 Dec;37(12):1774–1783. doi: 10.1002/art.1780371211. [DOI] [PubMed] [Google Scholar]
- Mattila L., Airola K., Ahonen M., Hietarinta M., Black C., Saarialho-Kere U., Kähäri V. M. Activation of tissue inhibitor of metalloproteinases-3 (TIMP-3) mRNA expression in scleroderma skin fibroblasts. J Invest Dermatol. 1998 Apr;110(4):416–421. doi: 10.1046/j.1523-1747.1998.00138.x. [DOI] [PubMed] [Google Scholar]
- Plumpton T. A., Clark I. M., Plumpton C., Calvin J., Cawston T. E. Development of an enzyme-linked immunosorbent assay to measure total TIMP-1 (free TIMP-1 and TIMP-1 in combination with matrix-metalloproteinases) and measurement of TIMP 1 and CRP in serum. Clin Chim Acta. 1995 Sep 15;240(2):137–154. doi: 10.1016/0009-8981(95)06137-7. [DOI] [PubMed] [Google Scholar]
- Scharffetter K., Lankat-Buttgereit B., Krieg T. Localization of collagen mRNA in normal and scleroderma skin by in-situ hybridization. Eur J Clin Invest. 1988 Feb;18(1):9–17. doi: 10.1111/j.1365-2362.1988.tb01158.x. [DOI] [PubMed] [Google Scholar]
- Takeda K., Hatamochi A., Ueki H., Nakata M., Oishi Y. Decreased collagenase expression in cultured systemic sclerosis fibroblasts. J Invest Dermatol. 1994 Sep;103(3):359–363. doi: 10.1111/1523-1747.ep12394936. [DOI] [PubMed] [Google Scholar]
- Uitto J., Bauer E. A., Eisen A. Z. Scleroderma: increased biosynthesis of triple-helical type I and type III procollagens associated with unaltered expression of collagenase by skin fibroblasts in culture. J Clin Invest. 1979 Oct;64(4):921–930. doi: 10.1172/JCI109558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yazawa N., Kikuchi K., Ihn H., Fujimoto M., Kubo M., Tamaki T., Tamaki K. Serum levels of tissue inhibitor of metalloproteinases 2 in patients with systemic sclerosis. J Am Acad Dermatol. 2000 Jan;42(1 Pt 1):70–75. doi: 10.1016/s0190-9622(00)90011-2. [DOI] [PubMed] [Google Scholar]


