Full Text
The Full Text of this article is available as a PDF (113.4 KB).
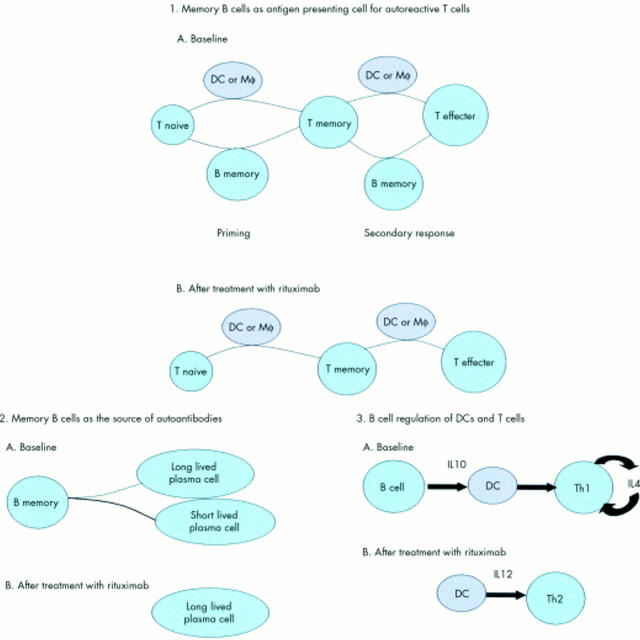
Figure 1 .
Possible effects of rituximab on human autoimmune disease.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arzoo K., Sadeghi S., Liebman H. A. Treatment of refractory antibody mediated autoimmune disorders with an anti-CD20 monoclonal antibody (rituximab). Ann Rheum Dis. 2002 Oct;61(10):922–924. doi: 10.1136/ard.61.10.922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berentsen S., Tjønnfjord G. E., Brudevold R., Gjertsen B. T., Langholm R., Løkkevik E., Sørbø J. H., Ulvestad E. Favourable response to therapy with the anti-CD20 monoclonal antibody rituximab in primary chronic cold agglutinin disease. Br J Haematol. 2001 Oct;115(1):79–83. doi: 10.1046/j.1365-2141.2001.03078.x. [DOI] [PubMed] [Google Scholar]
- Bussel J. B. Overview of idiopathic thrombocytopenic purpura: new approach to refractory patients. Semin Oncol. 2000 Dec;27(6 Suppl 12):91–98. [PubMed] [Google Scholar]
- Cartron Guillaume, Dacheux Laurent, Salles Gilles, Solal-Celigny Philippe, Bardos Pierre, Colombat Philippe, Watier Hervé. Therapeutic activity of humanized anti-CD20 monoclonal antibody and polymorphism in IgG Fc receptor FcgammaRIIIa gene. Blood. 2002 Feb 1;99(3):754–758. doi: 10.1182/blood.v99.3.754. [DOI] [PubMed] [Google Scholar]
- Chan O. T., Hannum L. G., Haberman A. M., Madaio M. P., Shlomchik M. J. A novel mouse with B cells but lacking serum antibody reveals an antibody-independent role for B cells in murine lupus. J Exp Med. 1999 May 17;189(10):1639–1648. doi: 10.1084/jem.189.10.1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan O. T., Madaio M. P., Shlomchik M. J. B cells are required for lupus nephritis in the polygenic, Fas-intact MRL model of systemic autoimmunity. J Immunol. 1999 Oct 1;163(7):3592–3596. [PubMed] [Google Scholar]
- Chan O. T., Madaio M. P., Shlomchik M. J. The central and multiple roles of B cells in lupus pathogenesis. Immunol Rev. 1999 Jun;169:107–121. doi: 10.1111/j.1600-065x.1999.tb01310.x. [DOI] [PubMed] [Google Scholar]
- Chan O., Shlomchik M. J. A new role for B cells in systemic autoimmunity: B cells promote spontaneous T cell activation in MRL-lpr/lpr mice. J Immunol. 1998 Jan 1;160(1):51–59. [PubMed] [Google Scholar]
- Clynes R. A., Towers T. L., Presta L. G., Ravetch J. V. Inhibitory Fc receptors modulate in vivo cytotoxicity against tumor targets. Nat Med. 2000 Apr;6(4):443–446. doi: 10.1038/74704. [DOI] [PubMed] [Google Scholar]
- Edwards J. C., Cambridge G. Sustained improvement in rheumatoid arthritis following a protocol designed to deplete B lymphocytes. Rheumatology (Oxford) 2001 Feb;40(2):205–211. doi: 10.1093/rheumatology/40.2.205. [DOI] [PubMed] [Google Scholar]
- Gausas J., Paterson P. Y., Day E. D., Dal Canto M. C. Intact B-cell activity is essential for complete expression of experimental allergic encephalomyelitis in Lewis rats. Cell Immunol. 1982 Sep 15;72(2):360–366. doi: 10.1016/0008-8749(82)90484-1. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Stawinski G. V., Yu P. B., Love S. D., Parker W., Davis R. D., Jr Hapten-induced primary and memory humoral responses are inhibited by the infusion of anti-CD20 monoclonal antibody (IDEC-C2B8, Rituximab). Clin Immunol. 2001 Feb;98(2):175–179. doi: 10.1006/clim.2000.4980. [DOI] [PubMed] [Google Scholar]
- Hayglass K. T., Naides S. J., Scott C. F., Jr, Benacerraf B., Sy M. S. T cell development in B cell-deficient mice. IV. The role of B cells as antigen-presenting cells in vivo. J Immunol. 1986 Feb 1;136(3):823–829. [PubMed] [Google Scholar]
- Janeway C. A., Jr, Ron J., Katz M. E. The B cell is the initiating antigen-presenting cell in peripheral lymph nodes. J Immunol. 1987 Feb 15;138(4):1051–1055. [PubMed] [Google Scholar]
- Leandro M. J., Edwards J. C. W., Cambridge G. Clinical outcome in 22 patients with rheumatoid arthritis treated with B lymphocyte depletion. Ann Rheum Dis. 2002 Oct;61(10):883–888. doi: 10.1136/ard.61.10.883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine T. D., Pestronk A. IgM antibody-related polyneuropathies: B-cell depletion chemotherapy using Rituximab. Neurology. 1999 May 12;52(8):1701–1704. doi: 10.1212/wnl.52.8.1701. [DOI] [PubMed] [Google Scholar]
- Li H., Shi F. D., He B., Bakheit M., Wahren B., Berglöf A., Sandstedt K., Link H. Experimental autoimmune myasthenia gravis induction in B cell-deficient mice. Int Immunol. 1998 Sep;10(9):1359–1365. doi: 10.1093/intimm/10.9.1359. [DOI] [PubMed] [Google Scholar]
- Liang Y., Buckley T. R., Tu L., Langdon S. D., Tedder T. F. Structural organization of the human MS4A gene cluster on Chromosome 11q12. Immunogenetics. 2001 Jul;53(5):357–368. doi: 10.1007/s002510100339. [DOI] [PubMed] [Google Scholar]
- Lin R. H., Mamula M. J., Hardin J. A., Janeway C. A., Jr Induction of autoreactive B cells allows priming of autoreactive T cells. J Exp Med. 1991 Jun 1;173(6):1433–1439. doi: 10.1084/jem.173.6.1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyons J. A., San M., Happ M. P., Cross A. H. B cells are critical to induction of experimental allergic encephalomyelitis by protein but not by a short encephalitogenic peptide. Eur J Immunol. 1999 Nov;29(11):3432–3439. doi: 10.1002/(SICI)1521-4141(199911)29:11<3432::AID-IMMU3432>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- Maloney D. G., Grillo-López A. J., White C. A., Bodkin D., Schilder R. J., Neidhart J. A., Janakiraman N., Foon K. A., Liles T. M., Dallaire B. K. IDEC-C2B8 (Rituximab) anti-CD20 monoclonal antibody therapy in patients with relapsed low-grade non-Hodgkin's lymphoma. Blood. 1997 Sep 15;90(6):2188–2195. [PubMed] [Google Scholar]
- Mamula M. J., Janeway C. A., Jr Do B cells drive the diversification of immune responses? Immunol Today. 1993 Apr;14(4):151–154. doi: 10.1016/0167-5699(93)90274-O. [DOI] [PubMed] [Google Scholar]
- Mamula M. J., Lin R. H., Janeway C. A., Jr, Hardin J. A. Breaking T cell tolerance with foreign and self co-immunogens. A study of autoimmune B and T cell epitopes of cytochrome c. J Immunol. 1992 Aug 1;149(3):789–795. [PubMed] [Google Scholar]
- Moulin V., Andris F., Thielemans K., Maliszewski C., Urbain J., Moser M. B lymphocytes regulate dendritic cell (DC) function in vivo: increased interleukin 12 production by DCs from B cell-deficient mice results in T helper cell type 1 deviation. J Exp Med. 2000 Aug 21;192(4):475–482. doi: 10.1084/jem.192.4.475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers K. J., Sprent J., Dougherty J. P., Ron Y. Synergy between encephalitogenic T cells and myelin basic protein-specific antibodies in the induction of experimental autoimmune encephalomyelitis. J Neuroimmunol. 1992 Nov;41(1):1–8. doi: 10.1016/0165-5728(92)90188-q. [DOI] [PubMed] [Google Scholar]
- Noorchashm H., Noorchashm N., Kern J., Rostami S. Y., Barker C. F., Naji A. B-cells are required for the initiation of insulitis and sialitis in nonobese diabetic mice. Diabetes. 1997 Jun;46(6):941–946. doi: 10.2337/diab.46.6.941. [DOI] [PubMed] [Google Scholar]
- Patel K., Berman J., Ferber A., Caro J. Refractory autoimmune thrombocytopenic purpura treatment with Rituximab. Am J Hematol. 2001 May;67(1):59–60. doi: 10.1002/ajh.1081. [DOI] [PubMed] [Google Scholar]
- Quartier P., Brethon B., Philippet P., Landman-Parker J., Le Deist F., Fischer A. Treatment of childhood autoimmune haemolytic anaemia with rituximab. Lancet. 2001 Nov 3;358(9292):1511–1513. doi: 10.1016/s0140-6736(01)06573-4. [DOI] [PubMed] [Google Scholar]
- Reff M. E., Carner K., Chambers K. S., Chinn P. C., Leonard J. E., Raab R., Newman R. A., Hanna N., Anderson D. R. Depletion of B cells in vivo by a chimeric mouse human monoclonal antibody to CD20. Blood. 1994 Jan 15;83(2):435–445. [PubMed] [Google Scholar]
- Rivera A., Chen C. C., Ron N., Dougherty J. P., Ron Y. Role of B cells as antigen-presenting cells in vivo revisited: antigen-specific B cells are essential for T cell expansion in lymph nodes and for systemic T cell responses to low antigen concentrations. Int Immunol. 2001 Dec;13(12):1583–1593. doi: 10.1093/intimm/13.12.1583. [DOI] [PubMed] [Google Scholar]
- Ron Y., De Baetselier P., Tzehoval E., Gordon J., Feldman M., Segal S. Defective induction of antigen-reactive proliferating T cells in B cell-deprived mice. II. Anti-mu treatment affects the initiation and recruitment of T cells. Eur J Immunol. 1983 Feb;13(2):167–171. doi: 10.1002/eji.1830130214. [DOI] [PubMed] [Google Scholar]
- Ron Y., Sprent J. T cell priming in vivo: a major role for B cells in presenting antigen to T cells in lymph nodes. J Immunol. 1987 May 1;138(9):2848–2856. [PubMed] [Google Scholar]
- Ron Y., de Baetselier P., Gordon J., Feldman M., Segal S. Impairment of antigen-specific T cell proliferative response in B cell suppressed mice. Adv Exp Med Biol. 1982;149:609–615. doi: 10.1007/978-1-4684-9066-4_84. [DOI] [PubMed] [Google Scholar]
- Sacchi S., Federico M., Dastoli G., Fiorani C., Vinci G., Clò V., Casolari B. Treatment of B-cell non-Hodgkin's lymphoma with anti CD 20 monoclonal antibody Rituximab. Crit Rev Oncol Hematol. 2001 Jan;37(1):13–25. doi: 10.1016/s1040-8428(00)00069-x. [DOI] [PubMed] [Google Scholar]
- Shlomchik M. J., Madaio M. P., Ni D., Trounstein M., Huszar D. The role of B cells in lpr/lpr-induced autoimmunity. J Exp Med. 1994 Oct 1;180(4):1295–1306. doi: 10.1084/jem.180.4.1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stasi R., Pagano A., Stipa E., Amadori S. Rituximab chimeric anti-CD20 monoclonal antibody treatment for adults with chronic idiopathic thrombocytopenic purpura. Blood. 2001 Aug 15;98(4):952–957. doi: 10.1182/blood.v98.4.952. [DOI] [PubMed] [Google Scholar]
- Svensson L., Jirholt J., Holmdahl R., Jansson L. B cell-deficient mice do not develop type II collagen-induced arthritis (CIA). Clin Exp Immunol. 1998 Mar;111(3):521–526. doi: 10.1046/j.1365-2249.1998.00529.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treon S. P., Anderson K. C. The use of rituximab in the treatment of malignant and nonmalignant plasma cell disorders. Semin Oncol. 2000 Dec;27(6 Suppl 12):79–85. [PubMed] [Google Scholar]
- Willenborg D. O., Prowse S. J. Immunoglobulin-deficient rats fail to develop experimental allergic encephalomyelitis. J Neuroimmunol. 1983 Oct;5(2):99–109. doi: 10.1016/0165-5728(83)90001-2. [DOI] [PubMed] [Google Scholar]
- Willenborg D. O., Sjollema P., Danta G. Immunoglobulin deficient rats as donors and recipients of effector cells of allergic encephalomyelitis. J Neuroimmunol. 1986 Apr;11(2):93–103. doi: 10.1016/0165-5728(86)90111-6. [DOI] [PubMed] [Google Scholar]