Abstract
Background: Interleukin 17 (IL17) is produced by activated T cells and has been implicated in the development of bone lesions and cartilage degradation in rheumatoid arthritis (RA).
Objective: To determine whether IL17, alone or together with tumour necrosis factor α (TNFα), induces cartilage destruction in vitro.
Methods: Fetal mouse metatarsals stripped of endogenous osteoclast precursors were used to study the effect of IL17 on cartilage degradation independently of osteoclastic resorption. Cartilage destruction was analysed histologically by Alcian blue staining.
Results: IL17 alone, up to 100 ng/ml, had no effect on the cartilage of fetal mouse metatarsals. IL17 (≥0.1 ng/ml), however, induced severe cartilage degradation when given together with TNFα (≥1 ng/ml). The cytokine combination decreased Alcian blue staining, a marker of proteoglycans, throughout the metatarsals and induced loss of the proliferating and early hypertrophic chondrocyte zones. TNFα alone also decreased Alcian blue staining, but not as dramatically as the cytokine combination. In addition, it did not induce loss of chondrocyte zones. Treatment with inhibitors of matrix metalloproteinase (MMP) activity and nitric oxide synthesis showed that MMP activity played a part in cartilage degradation, whereas nitric oxide production did not.
Conclusions: IL17, together with TNFα, induced cartilage degradation in fetal mouse metatarsals in vitro. IL17 may, therefore, participate in the development of cartilage destruction associated with RA by enhancing the effects of TNFα and may provide a potential therapeutic target.
Full Text
The Full Text of this article is available as a PDF (246.5 KB).
Figure 1 .
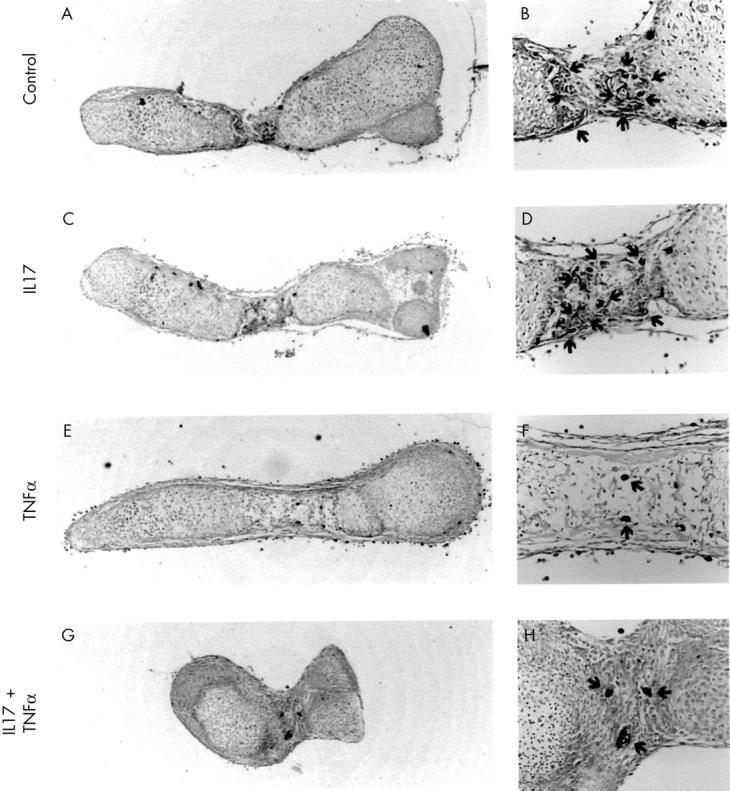
Histological staining of 19 day old fetal mouse metatarsals cultured in the absence or presence of IL17, TNFα, or a combination of the two cytokines. 19 Day old fetal mouse metatarsals were cultured for seven days in the absence (A, B) or presence of IL17 (100 ng/ml) (C, D), TNFα (100 ng/ml) (E, F), or a combination of the two cytokines (G, H). The presence of mononuclear and multinuclear osteoclasts was visualised using TRAP staining. Magnification: A, C, E, G x40; B, D, F, H x200. Cultures were performed in quintuplicate (n=5).
Figure 2 .
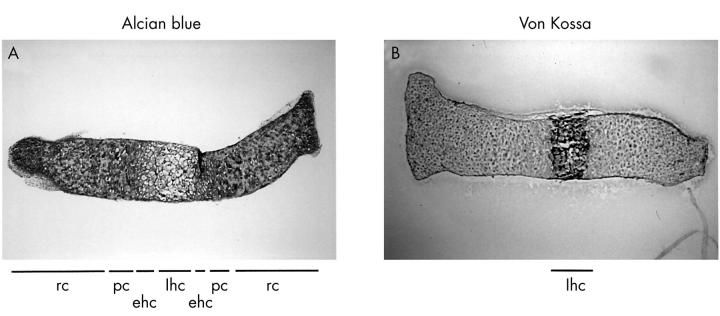
Zones of chondrocyte differentiation in fetal mouse metatarsals. 17 Day old fetal mouse metatarsals were fixed directly after isolation and stained for Alcian blue (A) or von Kossa (B). Four different zones of chondrocyte differentiation are shown: lhc, mineralised late hypertrophic chondrocytes (black von Kossa staining); ehc, unmineralised early hypertrophic chondrocytes; pc, proliferating chondrocytes; rc, resting chondrocytes. Magnification x40.
Figure 3 .
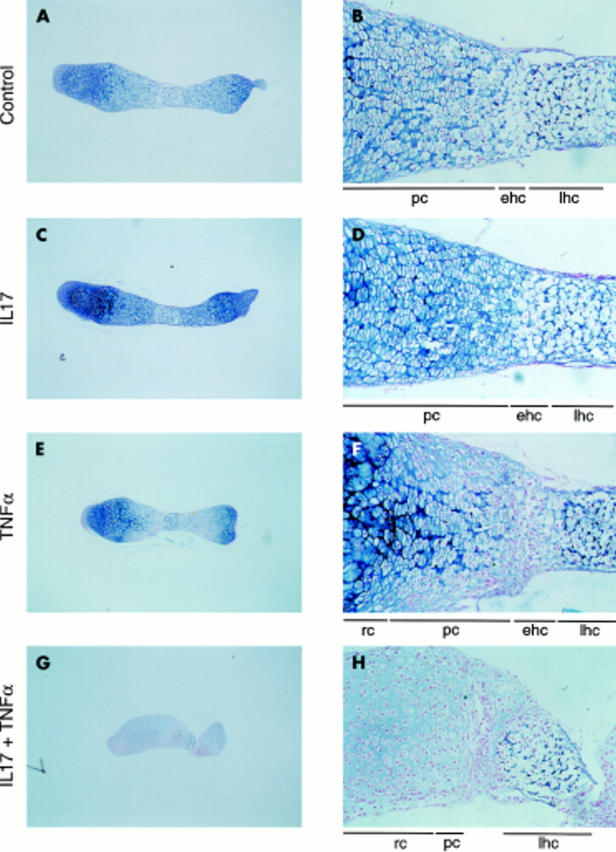
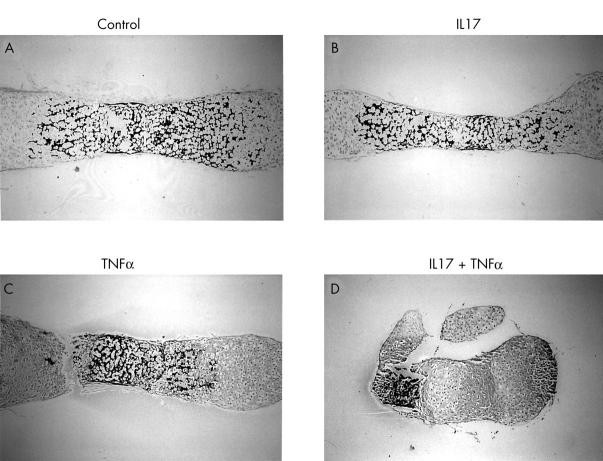
Effect of IL17, TNFα, and a combination of the two cytokines on Alcian blue staining. Osteoclast-free 17 day old fetal mouse metatarsals were cultured for 11 days in the absence (A, B) or presence of IL17 (50 ng/ml) (C, D), TNFα (50 ng/ml) (E, F), or a combination of the two cytokines (G, H) and stained with Alcian blue. lhc, late hypertrophic chondrocytes; ehc, early hypertrophic chondrocytes; pc, proliferating chondrocytes; rc, resting chondrocytes. Magnification: A, C, E, G x40; B, D, F, H x200. Cultures were performed in quadruplicate (n=4).
Figure 4 .
Effect of IL17, TNFα, and a combination of the two cytokines on von Kossa staining. Osteoclast-free 17 day old fetal mouse metatarsals were cultured for 11 days in the absence (A) or presence of IL17 (50 ng/ml) (B), TNFα (50 ng/ml) (C), or a combination of the two cytokines (D) and stained with von Kossa. Magnification x100. Cultures were performed in quadruplicate (n=4).
Figure 5 .
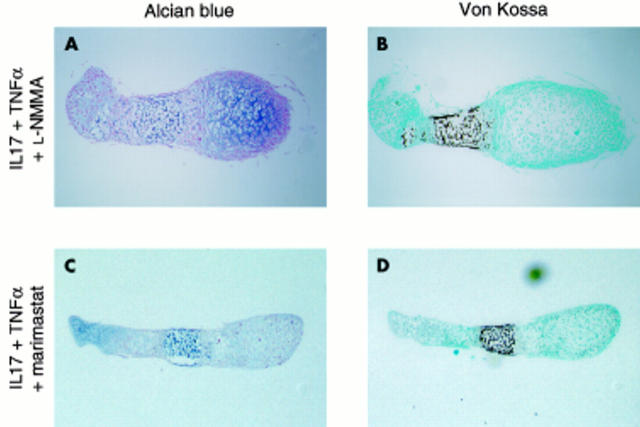
Effect of an NO synthesis inhibitor and an MMP inhibitor on cartilage destruction induced by IL17 + TNFα. Osteoclast-free 17 day old fetal mouse metatarsals were treated with IL17 (100 ng/ml) + TNFα (100 ng/ml) for 11 days in the presence of the nitric oxide synthesis inhibitor L-NMMA (1 mM) (A, B) or the MMP inhibitor marimastat (10-6 M) (C, D) and stained with Alcian blue (A, C) or von Kossa (B, D). Marimastat was dissolved in DMSO and the final concentration of DMSO in culture medium was 0.05%. This DMSO concentration had no effect on the histological picture of control and cytokine treated cultures (data not shown). DMSO, dimethylsulphoxide. Magnification x40. Cultures were performed in quadruplicate (n=4).
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aarvak T., Chabaud M., Miossec P., Natvig J. B. IL-17 is produced by some proinflammatory Th1/Th0 cells but not by Th2 cells. J Immunol. 1999 Feb 1;162(3):1246–1251. [PubMed] [Google Scholar]
- Attur M. G., Patel R. N., Abramson S. B., Amin A. R. Interleukin-17 up-regulation of nitric oxide production in human osteoarthritis cartilage. Arthritis Rheum. 1997 Jun;40(6):1050–1053. doi: 10.1002/art.1780400609. [DOI] [PubMed] [Google Scholar]
- Blavier L., Delaissé J. M. Matrix metalloproteinases are obligatory for the migration of preosteoclasts to the developing marrow cavity of primitive long bones. J Cell Sci. 1995 Dec;108(Pt 12):3649–3659. doi: 10.1242/jcs.108.12.3649. [DOI] [PubMed] [Google Scholar]
- Bramhall S. R. The matrix metalloproteinases and their inhibitors in pancreatic cancer. From molecular science to a clinical application. Int J Pancreatol. 1997 Feb;21(1):1–12. doi: 10.1007/BF02785914. [DOI] [PubMed] [Google Scholar]
- Breedveld F. C. New insights in the pathogenesis of rheumatoid arthritis. J Rheumatol Suppl. 1998 Jul;53:3–7. [PubMed] [Google Scholar]
- Bresnihan B. Pathogenesis of joint damage in rheumatoid arthritis. J Rheumatol. 1999 Mar;26(3):717–719. [PubMed] [Google Scholar]
- Burger E. H., Van der Meer J. W., van de Gevel J. S., Gribnau J. C., Thesingh G. W., van Furth R. In vitro formation of osteoclasts from long-term cultures of bone marrow mononuclear phagocytes. J Exp Med. 1982 Dec 1;156(6):1604–1614. doi: 10.1084/jem.156.6.1604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai L., Yin J. P., Starovasnik M. A., Hogue D. A., Hillan K. J., Mort J. S., Filvaroff E. H. Pathways by which interleukin 17 induces articular cartilage breakdown in vitro and in vivo. Cytokine. 2001 Oct 7;16(1):10–21. doi: 10.1006/cyto.2001.0939. [DOI] [PubMed] [Google Scholar]
- Cawston T. Matrix metalloproteinases and TIMPs: properties and implications for the rheumatic diseases. Mol Med Today. 1998 Mar;4(3):130–137. doi: 10.1016/s1357-4310(97)01192-1. [DOI] [PubMed] [Google Scholar]
- Chabaud M., Aarvak T., Garnero P., Natvig J. B., Miossec P. Potential contribution of IL-17-producing Th(1)cells to defective repair activity in joint inflammation: partial correction with Th(2)-promoting conditions. Cytokine. 2001 Jan 21;13(2):113–118. doi: 10.1006/cyto.2000.0811. [DOI] [PubMed] [Google Scholar]
- Chabaud M., Fossiez F., Taupin J. L., Miossec P. Enhancing effect of IL-17 on IL-1-induced IL-6 and leukemia inhibitory factor production by rheumatoid arthritis synoviocytes and its regulation by Th2 cytokines. J Immunol. 1998 Jul 1;161(1):409–414. [PubMed] [Google Scholar]
- Chabaud M., Garnero P., Dayer J. M., Guerne P. A., Fossiez F., Miossec P. Contribution of interleukin 17 to synovium matrix destruction in rheumatoid arthritis. Cytokine. 2000 Jul;12(7):1092–1099. doi: 10.1006/cyto.2000.0681. [DOI] [PubMed] [Google Scholar]
- Chabaud M., Lubberts E., Joosten L., van Den Berg W., Miossec P. IL-17 derived from juxta-articular bone and synovium contributes to joint degradation in rheumatoid arthritis. Arthritis Res. 2001 Jan 26;3(3):168–177. doi: 10.1186/ar294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chabaud M., Miossec P. The combination of tumor necrosis factor alpha blockade with interleukin-1 and interleukin-17 blockade is more effective for controlling synovial inflammation and bone resorption in an ex vivo model. Arthritis Rheum. 2001 Jun;44(6):1293–1303. doi: 10.1002/1529-0131(200106)44:6<1293::AID-ART221>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- Clancy R. M., Amin A. R., Abramson S. B. The role of nitric oxide in inflammation and immunity. Arthritis Rheum. 1998 Jul;41(7):1141–1151. doi: 10.1002/1529-0131(199807)41:7<1141::AID-ART2>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- Close D. R. Matrix metalloproteinase inhibitors in rheumatic diseases. Ann Rheum Dis. 2001 Nov;60 (Suppl 3):iii62–iii67. doi: 10.1136/ard.60.90003.iii62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudler J., Renggli-Zulliger N., Busso N., Lotz M., So A. Effect of interleukin 17 on proteoglycan degradation in murine knee joints. Ann Rheum Dis. 2000 Jul;59(7):529–532. doi: 10.1136/ard.59.7.529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudler J., So A. K. T cells and related cytokines. Curr Opin Rheumatol. 1998 May;10(3):207–211. doi: 10.1097/00002281-199805000-00009. [DOI] [PubMed] [Google Scholar]
- Ellis A. J., Curry V. A., Powell E. K., Cawston T. E. The prevention of collagen breakdown in bovine nasal cartilage by TIMP, TIMP-2 and a low molecular weight synthetic inhibitor. Biochem Biophys Res Commun. 1994 May 30;201(1):94–101. doi: 10.1006/bbrc.1994.1673. [DOI] [PubMed] [Google Scholar]
- Fahmi H., Di Battista J. A., Pelletier J. P., Mineau F., Ranger P., Martel-Pelletier J. Peroxisome proliferator--activated receptor gamma activators inhibit interleukin-1beta-induced nitric oxide and matrix metalloproteinase 13 production in human chondrocytes. Arthritis Rheum. 2001 Mar;44(3):595–607. doi: 10.1002/1529-0131(200103)44:3<595::AID-ANR108>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- Fox D. A. The role of T cells in the immunopathogenesis of rheumatoid arthritis: new perspectives. Arthritis Rheum. 1997 Apr;40(4):598–609. doi: 10.1002/art.1780400403. [DOI] [PubMed] [Google Scholar]
- Furst D. E., Keystone E. C., Breedveld F. C., Kalden J. R., Smolen J. S., Antoni C. E., Burmester G. R., Crofford L. J., Kavanaugh A. Updated consensus statement on tumour necrosis factor blocking agents for the treatment of rheumatoid arthritis and other rheumatic diseases (April 2001). Ann Rheum Dis. 2001 Nov;60 (Suppl 3):iii2–iii5. doi: 10.1136/ard.60.90003.iii2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honorati M. C., Meliconi R., Pulsatelli L., Canè S., Frizziero L., Facchini A. High in vivo expression of interleukin-17 receptor in synovial endothelial cells and chondrocytes from arthritis patients. Rheumatology (Oxford) 2001 May;40(5):522–527. doi: 10.1093/rheumatology/40.5.522. [DOI] [PubMed] [Google Scholar]
- Hukkanen M., Corbett S. A., Platts L. A., Konttinen Y. T., Santavirta S., Hughes S. P., Polak J. M. Nitric oxide in the local host reaction to total hip replacement. Clin Orthop Relat Res. 1998 Jul;(352):53–65. [PubMed] [Google Scholar]
- Jovanovic D. V., Di Battista J. A., Martel-Pelletier J., Reboul P., He Y., Jolicoeur F. C., Pelletier J. P. Modulation of TIMP-1 synthesis by antiinflammatory cytokines and prostaglandin E2 in interleukin 17 stimulated human monocytes/macrophages. J Rheumatol. 2001 Apr;28(4):712–718. [PubMed] [Google Scholar]
- Jovanovic D. V., Martel-Pelletier J., Di Battista J. A., Mineau F., Jolicoeur F. C., Benderdour M., Pelletier J. P. Stimulation of 92-kd gelatinase (matrix metalloproteinase 9) production by interleukin-17 in human monocyte/macrophages: a possible role in rheumatoid arthritis. Arthritis Rheum. 2000 May;43(5):1134–1144. doi: 10.1002/1529-0131(200005)43:5<1134::AID-ANR24>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- Kotake S., Udagawa N., Takahashi N., Matsuzaki K., Itoh K., Ishiyama S., Saito S., Inoue K., Kamatani N., Gillespie M. T. IL-17 in synovial fluids from patients with rheumatoid arthritis is a potent stimulator of osteoclastogenesis. J Clin Invest. 1999 May;103(9):1345–1352. doi: 10.1172/JCI5703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozaci L. D., Brown C. J., Adcocks C., Galloway A., Hollander A. P., Buttle D. J. Stromelysin 1, neutrophil collagenase, and collagenase 3 do not play major roles in a model of chondrocyte mediated cartilage breakdown. Mol Pathol. 1998 Oct;51(5):282–286. doi: 10.1136/mp.51.5.282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozaci L. D., Buttle D. J., Hollander A. P. Degradation of type II collagen, but not proteoglycan, correlates with matrix metalloproteinase activity in cartilage explant cultures. Arthritis Rheum. 1997 Jan;40(1):164–174. doi: 10.1002/art.1780400121. [DOI] [PubMed] [Google Scholar]
- Lotz M. The role of nitric oxide in articular cartilage damage. Rheum Dis Clin North Am. 1999 May;25(2):269–282. doi: 10.1016/s0889-857x(05)70067-3. [DOI] [PubMed] [Google Scholar]
- Lubberts E., Joosten L. A., van de Loo F. A., van den Gersselaar L. A., van den Berg W. B. Reduction of interleukin-17-induced inhibition of chondrocyte proteoglycan synthesis in intact murine articular cartilage by interleukin-4. Arthritis Rheum. 2000 Jun;43(6):1300–1306. doi: 10.1002/1529-0131(200006)43:6<1300::AID-ANR12>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- Löwik C. W., Nibbering P. H., van de Ruit M., Papapoulos S. E. Inducible production of nitric oxide in osteoblast-like cells and in fetal mouse bone explants is associated with suppression of osteoclastic bone resorption. J Clin Invest. 1994 Apr;93(4):1465–1472. doi: 10.1172/JCI117124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miossec P. Are T cells in rheumatoid synovium aggressors or bystanders? Curr Opin Rheumatol. 2000 May;12(3):181–185. doi: 10.1097/00002281-200005000-00004. [DOI] [PubMed] [Google Scholar]
- Moore B. A., Aznavoorian S., Engler J. A., Windsor L. J. Induction of collagenase-3 (MMP-13) in rheumatoid arthritis synovial fibroblasts. Biochim Biophys Acta. 2000 Oct 18;1502(2):307–318. doi: 10.1016/s0925-4439(00)00056-9. [DOI] [PubMed] [Google Scholar]
- Neidhart M., Fehr K., Pataki F., Michel B. A. The levels of memory (CD45RA-, RO+) CD4+ and CD8+ peripheral blood T-lymphocytes correlate with IgM rheumatoid factors in rheumatoid arthritis. Rheumatol Int. 1996;15(5):201–209. doi: 10.1007/BF00290522. [DOI] [PubMed] [Google Scholar]
- Nixon J. S., Bottomley K. M., Broadhurst M. J., Brown P. A., Johnson W. H., Lawton G., Marley J., Sedgwick A. D., Wilkinson S. E. Potent collagenase inhibitors prevent interleukin-1-induced cartilage degradation in vitro. Int J Tissue React. 1991;13(5):237–241. [PubMed] [Google Scholar]
- Shalom-Barak T., Quach J., Lotz M. Interleukin-17-induced gene expression in articular chondrocytes is associated with activation of mitogen-activated protein kinases and NF-kappaB. J Biol Chem. 1998 Oct 16;273(42):27467–27473. doi: 10.1074/jbc.273.42.27467. [DOI] [PubMed] [Google Scholar]
- Spriggs M. K. Interleukin-17 and its receptor. J Clin Immunol. 1997 Sep;17(5):366–369. doi: 10.1023/a:1027360106635. [DOI] [PubMed] [Google Scholar]
- Steward W. P. Marimastat (BB2516): current status of development. Cancer Chemother Pharmacol. 1999;43 (Suppl):S56–S60. doi: 10.1007/s002800051099. [DOI] [PubMed] [Google Scholar]
- Van Bezooijen R. L., Papapoulos S. E., Löwik C. W. Effect of interleukin-17 on nitric oxide production and osteoclastic bone resorption: is there dependency on nuclear factor-kappaB and receptor activator of nuclear factor kappaB (RANK)/RANK ligand signaling? Bone. 2001 Apr;28(4):378–386. doi: 10.1016/s8756-3282(00)00457-9. [DOI] [PubMed] [Google Scholar]
- Van bezooijen R. L., Farih-Sips H. C., Papapoulos S. E., Löwik C. W. Interleukin-17: A new bone acting cytokine in vitro. J Bone Miner Res. 1999 Sep;14(9):1513–1521. doi: 10.1359/jbmr.1999.14.9.1513. [DOI] [PubMed] [Google Scholar]
- Yocum D. E. T cells: pathogenic cells and therapeutic targets in rheumatoid arthritis. Semin Arthritis Rheum. 1999 Aug;29(1):27–35. doi: 10.1016/s0049-0172(99)80035-3. [DOI] [PubMed] [Google Scholar]
- Ziolkowska M., Koc A., Luszczykiewicz G., Ksiezopolska-Pietrzak K., Klimczak E., Chwalinska-Sadowska H., Maslinski W. High levels of IL-17 in rheumatoid arthritis patients: IL-15 triggers in vitro IL-17 production via cyclosporin A-sensitive mechanism. J Immunol. 2000 Mar 1;164(5):2832–2838. doi: 10.4049/jimmunol.164.5.2832. [DOI] [PubMed] [Google Scholar]
- van den Berg W. B., Joosten L. A., Kollias G., van De Loo F. A. Role of tumour necrosis factor alpha in experimental arthritis: separate activity of interleukin 1beta in chronicity and cartilage destruction. Ann Rheum Dis. 1999 Nov;58 (Suppl 1):I40–I48. doi: 10.1136/ard.58.2008.i40. [DOI] [PMC free article] [PubMed] [Google Scholar]