Abstract
Background: A possible relation between maternal-fetal microchimerism and autoimmune diseases with some similarities to chronic graft versus host disease (cGVHD) has been reported.
Objective: To investigate whether cells with male DNA exist in female patients with Sjögren's syndrome (SS) as SS has clinical features similar to those of cGVHD.
Methods: DNA was extracted from 27 samples of peripheral blood mononuclear cells (PBMC), 42 biopsy samples of labial salivary glands (LSG), and nine samples of bronchoalveolar lavage fluid (BALF) cells from 56 female patients with SS. The presence of male DNA was determined by nested polymerase chain reaction (PCR) and by fluorescence in situ hybridisation (FISH).
Results: Among 56 female patients with SS, 42 patients had at least one male child. Among those 42 patients, none of the 22 PBMC but 10/28 (36%) LSG samples tested positive by PCR for the Y chromosome-specific sequence (p=0.0013). The Y chromosome-specific sequence was not detected in the samples of LSG in 10 patients without SS. In the BALF samples 2/9 (22%) patients with SS tested positive by PCR. Cells containing the Y chromosome were shown to exist in all the LSG specimens from three female patients with SS by FISH.
Conclusions: Maternal-fetal microchimerism was shown for the first time to exist in the salivary glands and lungs of female patients with SS in this study. The presence of non-host cells in the inflammatory lesions but not in the peripheral blood suggests a possible role for maternal-fetal microchimerism in the pathogenesis of SS.
Full Text
The Full Text of this article is available as a PDF (206.1 KB).
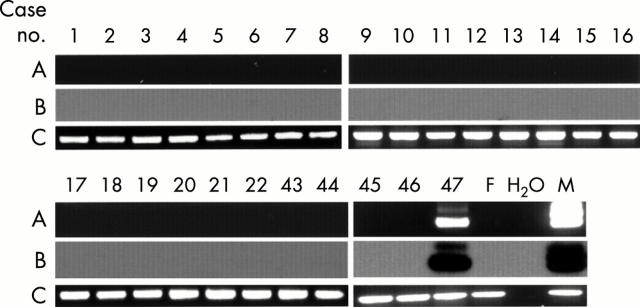
Figure 1 .
Detection of the Y chromosome-specific sequence in peripheral blood mononuclear cells from patients with SS with ethidium bromide staining (A) and its Southern blot analysis (B). Detection of ß-globin DNA by PCR with ethidium bromide staining is also shown as an internal control (C). Lane H2O, no DNA; lane F, female DNA control; lane M, male DNA control.
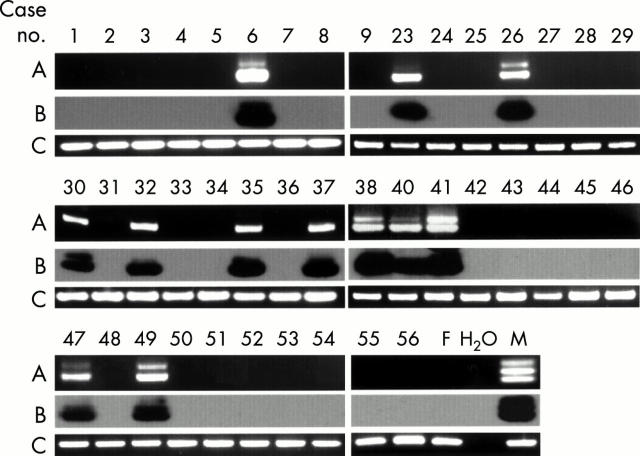
Figure 2 .
Detection of the Y chromosome-specific sequence in the biopsy specimens of the labial salivary glands from patients with SS. Other details are as in fig 1.
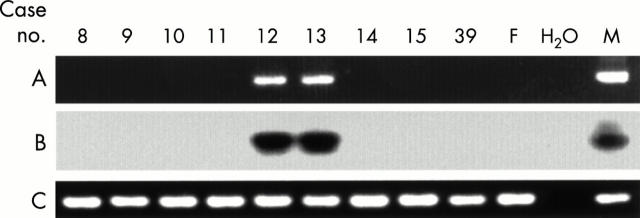
Figure 3 .
Detection of the Y chromosome-specific sequence in the pelleted cells from the bronchoalveolar lavage fluid from patients with SS. Other details are as in fig 1.
Figure 4 .
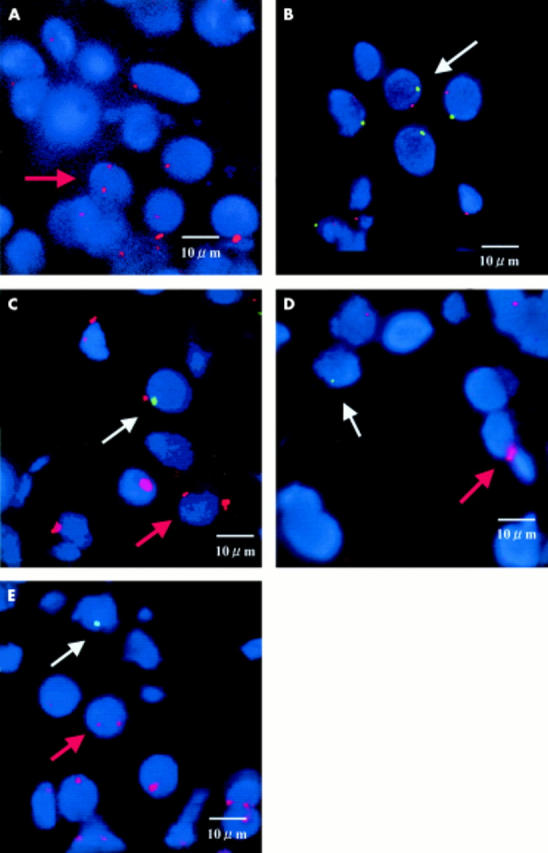
Fluorescence in situ hybridisation to detect the Y chromosome-containing cells in the biopsy specimens of the labial salivary glands. The Y chromosome probe was labelled with fluorescein (green signal), and the X chromosome probe was labelled with rhodamine red (red signal). (A) A female patient without SS (a negative control). Nucleus containing two X chromosomes (red signals). (B) A male patient with SS (a positive control). Nucleus containing one X chromosome (red signal) and one Y chromosome (green signal). (C, D, E) Female patients with SS who tested positive for the Y chromosome-specific sequence by PCR (Nos 40, 41, and 49, respectively) (study subjects). White arrow, nucleus containing one Y chromosome (green signal); red arrow, nucleus containing two X chromosomes (red signals).
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aractingi S., Berkane N., Bertheau P., Le Goué C., Dausset J., Uzan S., Carosella E. D. Fetal DNA in skin of polymorphic eruptions of pregnancy. Lancet. 1998 Dec 12;352(9144):1898–1901. doi: 10.1016/S0140-6736(98)05121-6. [DOI] [PubMed] [Google Scholar]
- Artlett C. M., Cox L. A., Jimenez S. A. Detection of cellular microchimerism of male or female origin in systemic sclerosis patients by polymerase chain reaction analysis of HLA-Cw antigens. Arthritis Rheum. 2000 May;43(5):1062–1067. doi: 10.1002/1529-0131(200005)43:5<1062::AID-ANR16>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- Artlett C. M., Ramos R., Jiminez S. A., Patterson K., Miller F. W., Rider L. G. Chimeric cells of maternal origin in juvenile idiopathic inflammatory myopathies. Childhood Myositis Heterogeneity Collaborative Group. Lancet. 2000 Dec 23;356(9248):2155–2156. doi: 10.1016/s0140-6736(00)03499-1. [DOI] [PubMed] [Google Scholar]
- Artlett C. M., Smith J. B., Jimenez S. A. Identification of fetal DNA and cells in skin lesions from women with systemic sclerosis. N Engl J Med. 1998 Apr 23;338(17):1186–1191. doi: 10.1056/NEJM199804233381704. [DOI] [PubMed] [Google Scholar]
- Bianchi D. W., Zickwolf G. K., Weil G. J., Sylvester S., DeMaria M. A. Male fetal progenitor cells persist in maternal blood for as long as 27 years postpartum. Proc Natl Acad Sci U S A. 1996 Jan 23;93(2):705–708. doi: 10.1073/pnas.93.2.705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gratwohl A. A., Gratwhol A. A., Moutsopoulos H. M., Chused T. M., Akizuki M., Wolf R. O., Sweet J. B., Deisseroth A. B. Sjögren-type syndrome after allogeneic bone-marrow transplantation. Ann Intern Med. 1977 Dec;87(6):703–706. doi: 10.7326/0003-4819-87-6-703. [DOI] [PubMed] [Google Scholar]
- Hiroki A., Nakamura S., Shinohara M., Gondo H., Ohyama Y., Hayashi S., Harada M., Niho Y., Oka M. A comparison of glandular involvement between chronic graft-versus-host disease and Sjögren's syndrome. Int J Oral Maxillofac Surg. 1996 Aug;25(4):298–307. doi: 10.1016/s0901-5027(06)80062-7. [DOI] [PubMed] [Google Scholar]
- Hiroki A., Nakamura S., Shinohara M., Oka M. Significance of oral examination in chronic graft-versus-host disease. J Oral Pathol Med. 1994 May;23(5):209–215. doi: 10.1111/j.1600-0714.1994.tb01115.x. [DOI] [PubMed] [Google Scholar]
- Homma M., Tojo T., Akizuki M., Yamagata H. Criteria for Sjögren's syndrome in Japan. Scand J Rheumatol Suppl. 1986;61:26–27. [PubMed] [Google Scholar]
- Jäger R. J., Anvret M., Hall K., Scherer G. A human XY female with a frame shift mutation in the candidate testis-determining gene SRY. Nature. 1990 Nov 29;348(6300):452–454. doi: 10.1038/348452a0. [DOI] [PubMed] [Google Scholar]
- Lawley T. J., Peck G. L., Moutsopoulos H. M., Gratwohl A. A., Deisseroth A. B. Scleroderma, Sjögren-like syndrome, and chronic graft-versus-host disease. Ann Intern Med. 1977 Dec;87(6):707–709. doi: 10.7326/0003-4819-87-6-707. [DOI] [PubMed] [Google Scholar]
- Lee T. H., Paglieroni T., Ohto H., Holland P. V., Busch M. P. Survival of donor leukocyte subpopulations in immunocompetent transfusion recipients: frequent long-term microchimerism in severe trauma patients. Blood. 1999 May 1;93(9):3127–3139. [PubMed] [Google Scholar]
- McMilin K. D., Johnson R. L. HLA homozygosity and the risk of related-donor transfusion-associated graft-versus-host disease. Transfus Med Rev. 1993 Jan;7(1):37–41. doi: 10.1016/s0887-7963(93)70031-0. [DOI] [PubMed] [Google Scholar]
- Miyashita Y., Ono M., Ono M., Ueki H., Kurasawa K. Y chromosome microchimerism in rheumatic autoimmune disease. Ann Rheum Dis. 2000 Aug;59(8):655–656. doi: 10.1136/ard.59.8.654b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murata H., Nakauchi H., Sumida T. Microchimerism in Japanese women patients with systemic sclerosis. Lancet. 1999 Jul 17;354(9174):220–220. doi: 10.1016/S0140-6736(99)00164-6. [DOI] [PubMed] [Google Scholar]
- Nakahori Y., Mitani K., Yamada M., Nakagome Y. A human Y-chromosome specific repeated DNA family (DYZ1) consists of a tandem array of pentanucleotides. Nucleic Acids Res. 1986 Oct 10;14(19):7569–7580. doi: 10.1093/nar/14.19.7569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson J. L., Furst D. E., Maloney S., Gooley T., Evans P. C., Smith A., Bean M. A., Ober C., Bianchi D. W. Microchimerism and HLA-compatible relationships of pregnancy in scleroderma. Lancet. 1998 Feb 21;351(9102):559–562. doi: 10.1016/S0140-6736(97)08357-8. [DOI] [PubMed] [Google Scholar]
- Reed A. M., Picornell Y. J., Harwood A., Kredich D. W. Chimerism in children with juvenile dermatomyositis. Lancet. 2000 Dec 23;356(9248):2156–2157. doi: 10.1016/S0140-6736(00)03500-5. [DOI] [PubMed] [Google Scholar]
- Scaletti Cristina, Vultaggio Alessandra, Bonifacio Stefania, Emmi Lorenzo, Torricelli Francesca, Maggi Enrico, Romagnani Sergio, Piccinni Marie-Pierre. Th2-oriented profile of male offspring T cells present in women with systemic sclerosis and reactive with maternal major histocompatibility complex antigens. Arthritis Rheum. 2002 Feb;46(2):445–450. doi: 10.1002/art.10049. [DOI] [PubMed] [Google Scholar]
- Sinclair A. H., Berta P., Palmer M. S., Hawkins J. R., Griffiths B. L., Smith M. J., Foster J. W., Frischauf A. M., Lovell-Badge R., Goodfellow P. N. A gene from the human sex-determining region encodes a protein with homology to a conserved DNA-binding motif. Nature. 1990 Jul 19;346(6281):240–244. doi: 10.1038/346240a0. [DOI] [PubMed] [Google Scholar]
- Tanaka A., Lindor K., Ansari A., Gershwin M. E. Fetal microchimerisms in the mother: immunologic implications. Liver Transpl. 2000 Mar;6(2):138–143. doi: 10.1002/lt.500060225. [DOI] [PubMed] [Google Scholar]
- Toda I., Kuwana M., Tsubota K., Kawakami Y. Lack of evidence for an increased microchimerism in the circulation of patients with Sjögren's syndrome. Ann Rheum Dis. 2001 Mar;60(3):248–253. doi: 10.1136/ard.60.3.248. [DOI] [PMC free article] [PubMed] [Google Scholar]