Abstract
Objective: To investigate if a difference exists between young and old mice in the response of articular cartilage to interleukin 1 (IL1) and transforming growth factor ß (TGFß) alone or in combination.
Methods: The interaction of IL1 and TGFß was studied in cartilage of young (three months) and old mice (18 months) both in vivo and in vitro. Therefore, IL1, TGFß, or IL1 together with TGFß was injected into the knee joints of mice on days 1, 3, and 5 before harvest of the patellae on day 6. Alternatively, isolated patellae were stimulated with IL1, TGFß, or IL1 together with TGFß in culture for 48 hours. Proteoglycan (PG) synthesis and nitric oxide (NO) production were measured.
Results: IL1 inhibited PG synthesis and increased NO production in cartilage of both young and old mice. On the other hand, TGFß stimulated PG synthesis and reduced NO production in both age groups. Importantly, TGFß was able to counteract IL1 mediated effects on PG synthesis and NO production in young but not in old mice.
Conclusions: Contrary to the findings in young mice, the cartilage of old animals does not antagonise IL1 effects via TGFß. This loss of responsiveness to the pivotal cytokine TGFß on effects of IL1 can be important in the initiation and progression of osteoarthritis (OA).
Full Text
The Full Text of this article is available as a PDF (126.4 KB).
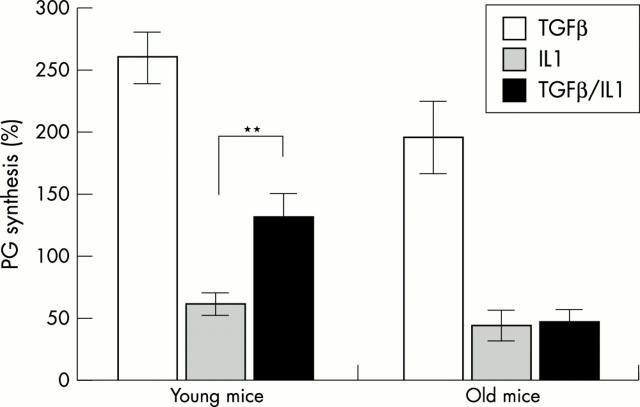
Figure 1 .
Synthesis of PG in patellar cartilage in old and young mice. Right knee joints of young mice (three months) and old mice (18 months) were injected on days 1,3, and 5 with TGFß (0.1 µg), IL1α (1 ng), or IL1 + TGFß. Left knee joints were injected with vehicle and served as internal controls (=100%). On day 6, patellae were isolated and PG synthesis was measured ex vivo by 35S-sulphate incorporation (two hours, 37°C). Each value represents the mean PG synthesis of five patellae. Results were statistically analysed by ANOVA; ** p<0.001.
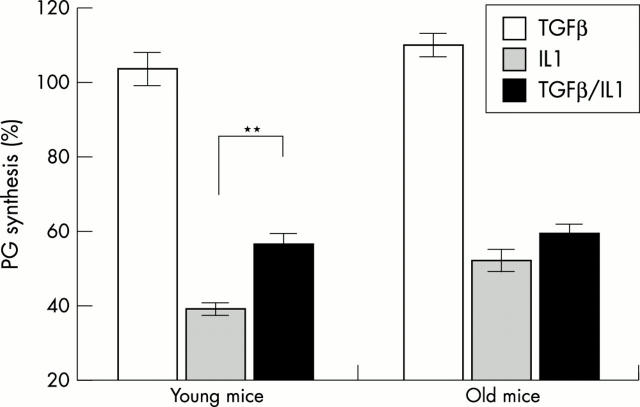
Figure 2 .
In vitro PG synthesis in patellar cartilage of young (three to five months) and old mice (12–21 months). Patellae were isolated and cultured in RPMI 1640 medium. Patellae were treated for 48 hours with IL1α (10 ng/ml), TGFß1 (10 ng/ml), a combination of IL1 + TGFß1, or were not treated. The tissue culture medium was changed every 24 hours. Synthesis of PG was measured via 35S-sulphate incorporation (two hours, 37°C). Incorporation levels of 35S were transformed to percentage compared with control treatment (=100%). Results were statistically analysed by ANOVA; **p<0.001.
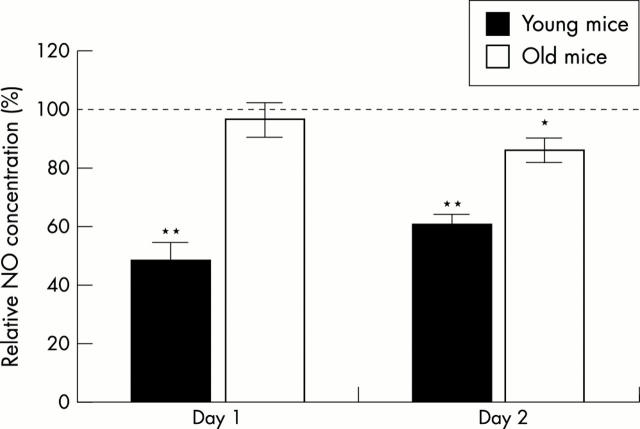
Figure 3 .
In vitro NO production after TGFß treatment by patellar cartilage of young (three to five months) and old mice (12 to 21 months). Patellae were isolated, placed in RPMI 1640 medium, and treated for 48 hours with TGFß (10 ng/ml) or were not treated. The tissue culture medium was changed every 24 hours. NO2- in the medium was measured after 24 and 48 hours by the Griess reaction. Production of NO by control treated patellae was stated as 100%. Results were statistically analysed by ANOVA; *p<0.01; ** p<0.001, significantly different from control treatment.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amin A. R., Abramson S. B. The role of nitric oxide in articular cartilage breakdown in osteoarthritis. Curr Opin Rheumatol. 1998 May;10(3):263–268. doi: 10.1097/00002281-199805000-00018. [DOI] [PubMed] [Google Scholar]
- Cawston T., Billington C., Cleaver C., Elliott S., Hui W., Koshy P., Shingleton B., Rowan A. The regulation of MMPs and TIMPs in cartilage turnover. Ann N Y Acad Sci. 1999 Jun 30;878:120–129. doi: 10.1111/j.1749-6632.1999.tb07678.x. [DOI] [PubMed] [Google Scholar]
- Chandrasekhar S., Harvey A. K. Transforming growth factor-beta is a potent inhibitor of IL-1 induced protease activity and cartilage proteoglycan degradation. Biochem Biophys Res Commun. 1988 Dec 30;157(3):1352–1359. doi: 10.1016/s0006-291x(88)81024-6. [DOI] [PubMed] [Google Scholar]
- Cicuttini F. M., Spector T. D. Osteoarthritis in the aged. Epidemiological issues and optimal management. Drugs Aging. 1995 May;6(5):409–420. doi: 10.2165/00002512-199506050-00007. [DOI] [PubMed] [Google Scholar]
- DiBattista J. A., Pelletier J. P., Zafarullah M., Fujimoto N., Obata K., Martel-Pelletier J. Coordinate regulation of matrix metalloproteases and tissue inhibitor of metalloproteinase expression in human synovial fibroblasts. J Rheumatol Suppl. 1995 Feb;43:123–128. [PubMed] [Google Scholar]
- Fava R., Olsen N., Keski-Oja J., Moses H., Pincus T. Active and latent forms of transforming growth factor beta activity in synovial effusions. J Exp Med. 1989 Jan 1;169(1):291–296. doi: 10.1084/jem.169.1.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldring M. B. The role of cytokines as inflammatory mediators in osteoarthritis: lessons from animal models. Connect Tissue Res. 1999;40(1):1–11. doi: 10.3109/03008209909005273. [DOI] [PubMed] [Google Scholar]
- Goldring M. B. The role of the chondrocyte in osteoarthritis. Arthritis Rheum. 2000 Sep;43(9):1916–1926. doi: 10.1002/1529-0131(200009)43:9<1916::AID-ANR2>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- Hamerman D. Aging and osteoarthritis: basic mechanisms. J Am Geriatr Soc. 1993 Jul;41(7):760–770. doi: 10.1111/j.1532-5415.1993.tb07469.x. [DOI] [PubMed] [Google Scholar]
- Imamura T., Takase M., Nishihara A., Oeda E., Hanai J., Kawabata M., Miyazono K. Smad6 inhibits signalling by the TGF-beta superfamily. Nature. 1997 Oct 9;389(6651):622–626. doi: 10.1038/39355. [DOI] [PubMed] [Google Scholar]
- Lafeber F. P., Vander Kraan P. M., Huber-Bruning O., Vanden Berg W. B., Bijlsma J. W. Osteoarthritic human cartilage is more sensitive to transforming growth factor beta than is normal cartilage. Br J Rheumatol. 1993 Apr;32(4):281–286. doi: 10.1093/rheumatology/32.4.281. [DOI] [PubMed] [Google Scholar]
- Lafeber F. P., van Roy H. L., van der Kraan P. M., van den Berg W. B., Bijlsma J. W. Transforming growth factor-beta predominantly stimulates phenotypically changed chondrocytes in osteoarthritic human cartilage. J Rheumatol. 1997 Mar;24(3):536–542. [PubMed] [Google Scholar]
- Lawrence R. C., Helmick C. G., Arnett F. C., Deyo R. A., Felson D. T., Giannini E. H., Heyse S. P., Hirsch R., Hochberg M. C., Hunder G. G. Estimates of the prevalence of arthritis and selected musculoskeletal disorders in the United States. Arthritis Rheum. 1998 May;41(5):778–799. doi: 10.1002/1529-0131(199805)41:5<778::AID-ART4>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- Lotz M., Kekow J., Carson D. A. Transforming growth factor-beta and cellular immune responses in synovial fluids. J Immunol. 1990 Jun 1;144(11):4189–4194. [PubMed] [Google Scholar]
- Moos V., Fickert S., Müller B., Weber U., Sieper J. Immunohistological analysis of cytokine expression in human osteoarthritic and healthy cartilage. J Rheumatol. 1999 Apr;26(4):870–879. [PubMed] [Google Scholar]
- Morales T. I., Joyce M. E., Sobel M. E., Danielpour D., Roberts A. B. Transforming growth factor-beta in calf articular cartilage organ cultures: synthesis and distribution. Arch Biochem Biophys. 1991 Aug 1;288(2):397–405. doi: 10.1016/0003-9861(91)90212-2. [DOI] [PubMed] [Google Scholar]
- Schlaak J. F., Pfers I., Meyer Zum Büschenfelde K. H., Märker-Hermann E. Different cytokine profiles in the synovial fluid of patients with osteoarthritis, rheumatoid arthritis and seronegative spondylarthropathies. Clin Exp Rheumatol. 1996 Mar-Apr;14(2):155–162. [PubMed] [Google Scholar]
- Serra R., Johnson M., Filvaroff E. H., LaBorde J., Sheehan D. M., Derynck R., Moses H. L. Expression of a truncated, kinase-defective TGF-beta type II receptor in mouse skeletal tissue promotes terminal chondrocyte differentiation and osteoarthritis. J Cell Biol. 1997 Oct 20;139(2):541–552. doi: 10.1083/jcb.139.2.541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefanovic-Racic M., Stadler J., Georgescu H. I., Evans C. H. Nitric oxide synthesis and its regulation by rabbit synoviocytes. J Rheumatol. 1994 Oct;21(10):1892–1898. [PubMed] [Google Scholar]
- Su S., Grover J., Roughley P. J., DiBattista J. A., Martel-Pelletier J., Pelletier J. P., Zafarullah M. Expression of the tissue inhibitor of metalloproteinases (TIMP) gene family in normal and osteoarthritic joints. Rheumatol Int. 1999;18(5-6):183–191. doi: 10.1007/s002960050083. [DOI] [PubMed] [Google Scholar]
- Tetlow L. C., Adlam D. J., Woolley D. E. Matrix metalloproteinase and proinflammatory cytokine production by chondrocytes of human osteoarthritic cartilage: associations with degenerative changes. Arthritis Rheum. 2001 Mar;44(3):585–594. doi: 10.1002/1529-0131(200103)44:3<585::AID-ANR107>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- Van de Loo F. A., Arntz O. J., Van den Berg W. B. Effect of interleukin 1 and leukaemia inhibitory factor on chondrocyte metabolism in articular cartilage from normal and interleukin-6-deficient mice: role of nitric oxide and IL-6 in the suppression of proteoglycan synthesis. Cytokine. 1997 Jul;9(7):453–462. doi: 10.1006/cyto.1997.0188. [DOI] [PubMed] [Google Scholar]
- Vodovotz Y., Bogdan C., Paik J., Xie Q. W., Nathan C. Mechanisms of suppression of macrophage nitric oxide release by transforming growth factor beta. J Exp Med. 1993 Aug 1;178(2):605–613. doi: 10.1084/jem.178.2.605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang X., Chen L., Xu X., Li C., Huang C., Deng C. X. TGF-beta/Smad3 signals repress chondrocyte hypertrophic differentiation and are required for maintaining articular cartilage. J Cell Biol. 2001 Apr 2;153(1):35–46. doi: 10.1083/jcb.153.1.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu H. J., Iaria J., Sizeland A. M. Smad7 differentially regulates transforming growth factor beta-mediated signaling pathways. J Biol Chem. 1999 Nov 5;274(45):32258–32264. doi: 10.1074/jbc.274.45.32258. [DOI] [PubMed] [Google Scholar]
- de Vries B. J., van den Berg W. B., Vitters E., van de Putte L. B. Quantitation of glycosaminoglycan metabolism in anatomically intact articular cartilage of the mouse patella: in vitro and in vivo studies with 35S-sulfate, 3H-glucosamine, and 3H-acetate. Rheumatol Int. 1986;6(6):273–281. doi: 10.1007/BF00541319. [DOI] [PubMed] [Google Scholar]
- ten Dijke P., Miyazono K., Heldin C. H. Signaling inputs converge on nuclear effectors in TGF-beta signaling. Trends Biochem Sci. 2000 Feb;25(2):64–70. doi: 10.1016/s0968-0004(99)01519-4. [DOI] [PubMed] [Google Scholar]
- van Beuningen H. M., Arntz O. J., van den Berg W. B. In vivo effects of interleukin-1 on articular cartilage. Prolongation of proteoglycan metabolic disturbances in old mice. Arthritis Rheum. 1991 May;34(5):606–615. doi: 10.1002/art.1780340513. [DOI] [PubMed] [Google Scholar]
- van Beuningen H. M., van der Kraan P. M., Arntz O. J., van den Berg W. B. In vivo protection against interleukin-1-induced articular cartilage damage by transforming growth factor-beta 1: age-related differences. Ann Rheum Dis. 1994 Sep;53(9):593–600. doi: 10.1136/ard.53.9.593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Beuningen H. M., van der Kraan P. M., Arntz O. J., van den Berg W. B. Protection from interleukin 1 induced destruction of articular cartilage by transforming growth factor beta: studies in anatomically intact cartilage in vitro and in vivo. Ann Rheum Dis. 1993 Mar;52(3):185–191. doi: 10.1136/ard.52.3.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Beuningen H. M., van der Kraan P. M., Arntz O. J., van den Berg W. B. Transforming growth factor-beta 1 stimulates articular chondrocyte proteoglycan synthesis and induces osteophyte formation in the murine knee joint. Lab Invest. 1994 Aug;71(2):279–290. [PubMed] [Google Scholar]
- van de Loo F. A., Arntz O. J., Otterness I. G., van den Berg W. B. Protection against cartilage proteoglycan synthesis inhibition by antiinterleukin 1 antibodies in experimental arthritis. J Rheumatol. 1992 Mar;19(3):348–356. [PubMed] [Google Scholar]
- van de Loo F. A., Arntz O. J., van Enckevort F. H., van Lent P. L., van den Berg W. B. Reduced cartilage proteoglycan loss during zymosan-induced gonarthritis in NOS2-deficient mice and in anti-interleukin-1-treated wild-type mice with unabated joint inflammation. Arthritis Rheum. 1998 Apr;41(4):634–646. doi: 10.1002/1529-0131(199804)41:4<634::AID-ART10>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- van de Loo F. A., Joosten L. A., van Lent P. L., Arntz O. J., van den Berg W. B. Role of interleukin-1, tumor necrosis factor alpha, and interleukin-6 in cartilage proteoglycan metabolism and destruction. Effect of in situ blocking in murine antigen- and zymosan-induced arthritis. Arthritis Rheum. 1995 Feb;38(2):164–172. doi: 10.1002/art.1780380204. [DOI] [PubMed] [Google Scholar]
- van den Berg W. B. Anti-cytokine therapy in chronic destructive arthritis. Arthritis Res. 2000 Nov 10;3(1):18–26. doi: 10.1186/ar136. [DOI] [PMC free article] [PubMed] [Google Scholar]