Abstract
Objective: To determine the serum levels of soluble adhesion molecules in patients with juvenile idiopathic arthritis (JIA), and to determine whether the levels of these molecules differ between active disease and remission in the same JIA subtype, and whether differences in these levels exist between controls and the three JIA subtypes.
Methods: The serum levels of soluble E-selectin (sE-selectin) and soluble intercellular adhesion molecule-1 (sICAM-1) were determined by enzyme linked immunosorbent assay (ELISA) in 40 patients with JIA (12 systemic, 13 polyarticular, and 15 oligoarticular) who had active disease or were in clinical remission and 16 healthy controls. Differences in the levels of adhesion molecules of the same JIA subtype during different disease activity were determined by the pairedt test, and differences between the disease and control groups were calculated by one way analysis of variance. A value p<0.01 was considered significant.
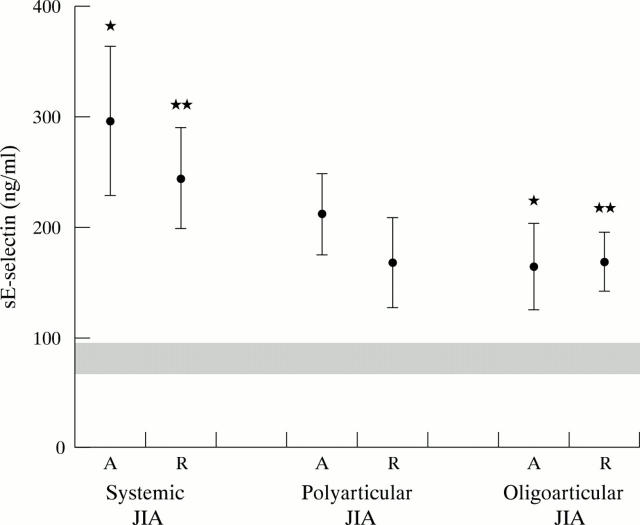
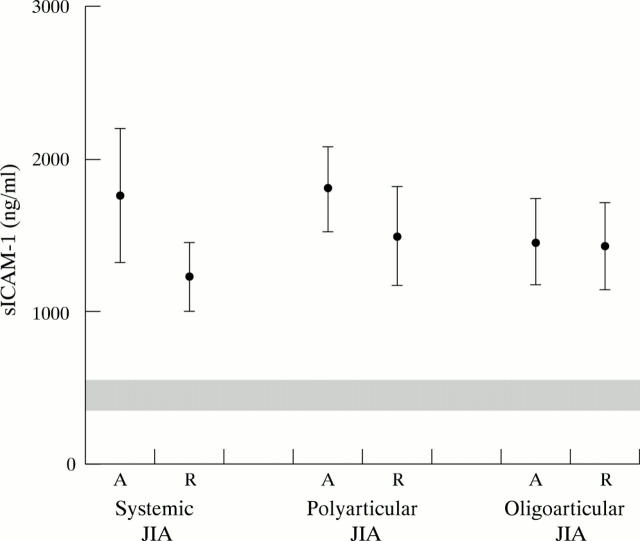
Results: During the same disease stage (active or in remission), systemic JIA was associated with a significantly higher sE-selectin level than the oligoarticular JIA subtype, whereas this was not found for sICAM-1. Although the mean levels of sE-selectin and sICAM-1 in active systemic and polyarticular JIA were higher than those in remission, this did not reach statistical significance. The levels of sE-selectin and sICAM-1 of the three JIA subtypes, in both the active stage and clinical remission, were still significantly higher than in normal controls.
Conclusions: Systemic JIA is associated with a higher sE-selectin level than oligoarticular JIA both in active disease and in clinical remission. This may explain why the morbidity of systemic JIA is greater than that of oligoarticular JIA—namely, owing to increased endothelial cell activation. As significantly higher levels of sE-selectin and sICAM-1 were found in the active and remission stages of the three JIA subtypes compared with those in the control group, JIA may recur even when clinical remission has been achieved.
Full Text
The Full Text of this article is available as a PDF (130.0 KB).
Figure 1 .
The serum levels of sE-selectin in different JIA subtypes and in active disease and remission. Mean values of sE-selectin in the different subtypes are represented by black circles, with ±2SEM represented by the upper and lower confidence bars. The range of normal control (mean ±2SEM) is represented by the shaded band. A, active disease; R, remission. *In active disease, systemic v oligoarticular, p<0.01; **in remission, systemic v oligoarticular, p<0.01.
Figure 2 .
Serum levels of sICAM-1 in different JIA subtypes and in active disease and remission. Mean values of sICAM-1 in the different subtypes are represented by black circles, with ±2SEM represented by the upper and lower confidence bars. The range of normal control (mean ±2SEM) is represented by the shaded band. A, active disease; R, remission.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aoki S., Imai K., Yachi A. Soluble intercellular adhesion molecule-1 (ICAM-1) antigen in patients with rheumatoid arthritis. Scand J Immunol. 1993 Nov;38(5):485–490. doi: 10.1111/j.1365-3083.1993.tb02592.x. [DOI] [PubMed] [Google Scholar]
- Bevilacqua M. P., Stengelin S., Gimbrone M. A., Jr, Seed B. Endothelial leukocyte adhesion molecule 1: an inducible receptor for neutrophils related to complement regulatory proteins and lectins. Science. 1989 Mar 3;243(4895):1160–1165. doi: 10.1126/science.2466335. [DOI] [PubMed] [Google Scholar]
- Bloom B. J., Miller L. C., Tucker L. B., Schaller J. G., Blier P. R. Soluble adhesion molecules in juvenile rheumatoid arthritis. J Rheumatol. 1999 Sep;26(9):2044–2048. [PubMed] [Google Scholar]
- Buck C. A. Immunoglobulin superfamily: structure, function and relationship to other receptor molecules. Semin Cell Biol. 1992 Jun;3(3):179–188. doi: 10.1016/s1043-4682(10)80014-5. [DOI] [PubMed] [Google Scholar]
- Dustin M. L., Rothlein R., Bhan A. K., Dinarello C. A., Springer T. A. Induction by IL 1 and interferon-gamma: tissue distribution, biochemistry, and function of a natural adherence molecule (ICAM-1). J Immunol. 1986 Jul 1;137(1):245–254. [PubMed] [Google Scholar]
- Gearing A. J., Newman W. Circulating adhesion molecules in disease. Immunol Today. 1993 Oct;14(10):506–512. doi: 10.1016/0167-5699(93)90267-O. [DOI] [PubMed] [Google Scholar]
- Kavanaugh A. F., Davis L. S., Nichols L. A., Norris S. H., Rothlein R., Scharschmidt L. A., Lipsky P. E. Treatment of refractory rheumatoid arthritis with a monoclonal antibody to intercellular adhesion molecule 1. Arthritis Rheum. 1994 Jul;37(7):992–999. doi: 10.1002/art.1780370703. [DOI] [PubMed] [Google Scholar]
- Koch A. E., Turkiewicz W., Harlow L. A., Pope R. M. Soluble E-selectin in arthritis. Clin Immunol Immunopathol. 1993 Oct;69(1):29–35. doi: 10.1006/clin.1993.1146. [DOI] [PubMed] [Google Scholar]
- Kolopp-Sarda M. N., Guillemin F., Chary-Valckenaere I., Béné M. C., Pourel J., Faure G. C. Longitudinal study of rheumatoid arthritis patients discloses sustained elevated serum levels of soluble CD106 (V-CAM). Clin Exp Rheumatol. 2001 Mar-Apr;19(2):165–170. [PubMed] [Google Scholar]
- Larsen A., Dale K., Eek M. Radiographic evaluation of rheumatoid arthritis and related conditions by standard reference films. Acta Radiol Diagn (Stockh) 1977 Jul;18(4):481–491. doi: 10.1177/028418517701800415. [DOI] [PubMed] [Google Scholar]
- Lasky L. A. Selectins: interpreters of cell-specific carbohydrate information during inflammation. Science. 1992 Nov 6;258(5084):964–969. doi: 10.1126/science.1439808. [DOI] [PubMed] [Google Scholar]
- Laucella S. A., Gaddi E., Balbaryski J., Giraudi V., Cuttica R. J. Soluble intercellular adhesion molecule-1 in paediatric connective tissue diseases. Acta Paediatr. 1999 Apr;88(4):399–403. doi: 10.1080/08035259950169765. [DOI] [PubMed] [Google Scholar]
- Lozada C., Levin R. I., Huie M., Hirschhorn R., Naime D., Whitlow M., Recht P. A., Golden B., Cronstein B. N. Identification of C1q as the heat-labile serum cofactor required for immune complexes to stimulate endothelial expression of the adhesion molecules E-selectin and intercellular and vascular cell adhesion molecules 1. Proc Natl Acad Sci U S A. 1995 Aug 29;92(18):8378–8382. doi: 10.1073/pnas.92.18.8378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason J. C., Kapahi P., Haskard D. O. Detection of increased levels of circulating intercellular adhesion molecule 1 in some patients with rheumatoid arthritis but not in patients with systemic lupus erythematosus. Lack of correlation with levels of circulating vascular cell adhesion molecule 1. Arthritis Rheum. 1993 Apr;36(4):519–527. doi: 10.1002/art.1780360412. [DOI] [PubMed] [Google Scholar]
- Mojcik C. F., Shevach E. M. Adhesion molecules: a rheumatologic perspective. Arthritis Rheum. 1997 Jun;40(6):991–1004. doi: 10.1002/art.1780400602. [DOI] [PubMed] [Google Scholar]
- Petty R. E., Southwood T. R., Baum J., Bhettay E., Glass D. N., Manners P., Maldonado-Cocco J., Suarez-Almazor M., Orozco-Alcala J., Prieur A. M. Revision of the proposed classification criteria for juvenile idiopathic arthritis: Durban, 1997. J Rheumatol. 1998 Oct;25(10):1991–1994. [PubMed] [Google Scholar]
- Pinals R. S., Masi A. T., Larsen R. A. Preliminary criteria for clinical remission in rheumatoid arthritis. Arthritis Rheum. 1981 Oct;24(10):1308–1315. doi: 10.1002/art.1780241012. [DOI] [PubMed] [Google Scholar]
- Pitzalis C., Kingsley G., Panayi G. Adhesion molecules in rheumatoid arthritis: role in the pathogenesis and prospects for therapy. Ann Rheum Dis. 1994 May;53(5):287–288. doi: 10.1136/ard.53.5.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritchie D. M., Boyle J. A., McInnes J. M., Jasani M. K., Dalakos T. G., Grieveson P., Buchanan W. W. Clinical studies with an articular index for the assessment of joint tenderness in patients with rheumatoid arthritis. Q J Med. 1968 Jul;37(147):393–406. [PubMed] [Google Scholar]
- Rothlein R., Czajkowski M., O'Neill M. M., Marlin S. D., Mainolfi E., Merluzzi V. J. Induction of intercellular adhesion molecule 1 on primary and continuous cell lines by pro-inflammatory cytokines. Regulation by pharmacologic agents and neutralizing antibodies. J Immunol. 1988 Sep 1;141(5):1665–1669. [PubMed] [Google Scholar]
- Rothschild B. M., Masi A. T. Pathogenesis of rheumatoid arthritis: a vascular hypothesis. Semin Arthritis Rheum. 1982 Aug;12(1):11–31. doi: 10.1016/0049-0172(82)90020-8. [DOI] [PubMed] [Google Scholar]
- Scola M. P., Imagawa T., Boivin G. P., Giannini E. H., Glass D. N., Hirsch R., Grom A. A. Expression of angiogenic factors in juvenile rheumatoid arthritis: correlation with revascularization of human synovium engrafted into SCID mice. Arthritis Rheum. 2001 Apr;44(4):794–801. doi: 10.1002/1529-0131(200104)44:4<794::AID-ANR135>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- Springer T. A. Traffic signals for lymphocyte recirculation and leukocyte emigration: the multistep paradigm. Cell. 1994 Jan 28;76(2):301–314. doi: 10.1016/0092-8674(94)90337-9. [DOI] [PubMed] [Google Scholar]
- Vestweber D. Selectins: cell surface lectins which mediate the binding of leukocytes to endothelial cells. Semin Cell Biol. 1992 Jun;3(3):211–220. doi: 10.1016/s1043-4682(10)80017-0. [DOI] [PubMed] [Google Scholar]
- Wellicome S. M., Kapahi P., Mason J. C., Lebranchu Y., Yarwood H., Haskard D. O. Detection of a circulating form of vascular cell adhesion molecule-1: raised levels in rheumatoid arthritis and systemic lupus erythematosus. Clin Exp Immunol. 1993 Jun;92(3):412–418. doi: 10.1111/j.1365-2249.1993.tb03413.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yellin M. J., Brett J., Baum D., Matsushima A., Szabolcs M., Stern D., Chess L. Functional interactions of T cells with endothelial cells: the role of CD40L-CD40-mediated signals. J Exp Med. 1995 Dec 1;182(6):1857–1864. doi: 10.1084/jem.182.6.1857. [DOI] [PMC free article] [PubMed] [Google Scholar]