Abstract
Objectives: Even though clinical findings support the idea that hip osteoarthritis (OA) is associated with increased bone mineral density (BMD), the subject remains controversial. This study was therefore initiated to investigate the relation between the severity of hip OA and femoral and calcaneal BMD.
Methods: On the basis of the American College of Rheumatology criteria on classification of OA of the hip, 27 men (aged 47–64 years) with unilateral or bilateral hip OA and 30 age matched randomly selected healthy men were studied. Plain radiographs were graded using Li's scale from 0 (no OA) to 4 (severe OA). According to the side of the highest radiographic score from the patients with clinical hip OA, 29.6% had grade 1, 29.6% grade 2, and 40.8% grade 3 OA. Bone mineral content (BMC), areal BMD (BMDareal), and bone dimensions (area and width) were measured by dual x ray absorptiometry at the proximal femur. BMDareal of the calcaneus was measured from the central area of the bone. Volumetric measurements from magnetic resonance images of the femoral neck were used to create a BMD measure that was corrected for the femoral neck volume (BMDmri).
Results: There were no differences in weight, or body mass index between the study groups. There were no significant BMDareal differences in any of the subregions of the proximal femur (femoral neck and trochanter) or calcaneus between the OA and control groups. Neither did the BMDmri of the femoral neck differ between the groups. However, the BMC of the femoral neck was 18% higher (p<0.01) in patients with OA than in controls. Similarly femoral neck bone width and volume were 9% and 18% respectively higher (p<0.001) in patients with OA.
Conclusions: The results suggest that men with hip OA have larger femoral neck size and consequently higher BMC than healthy controls matched for age and sex. There is no significant difference in femoral neck BMD (BMDareal or BMDmri) between the groups. Furthermore, increased BMDareal was not found in the peripheral skeleton. These findings suggest that hip OA is not associated with an increase in BMDareal in the femoral neck. However, the increase in BMC and bone size in patients with hip OA may play a part in the pathogenesis of the disease.
Full Text
The Full Text of this article is available as a PDF (143.9 KB).
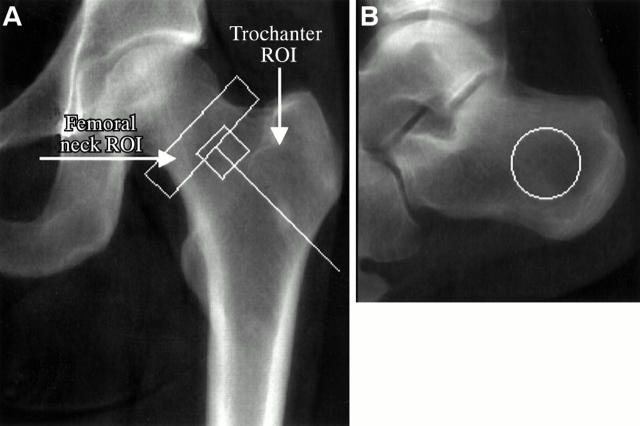
Figure 1 .
Location of the regions of interest (ROIs) in the proximal femur (A) and calcaneus (B).
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alhava E. M., Kettunen K., Karjalainen P. Bone mineral in patients with osteoarthrosis of the hip. Acta Orthop Scand. 1975 Nov;46(5):709–715. doi: 10.3109/17453677508989256. [DOI] [PubMed] [Google Scholar]
- Altman R., Alarcón G., Appelrouth D., Bloch D., Borenstein D., Brandt K., Brown C., Cooke T. D., Daniel W., Feldman D. The American College of Rheumatology criteria for the classification and reporting of osteoarthritis of the hip. Arthritis Rheum. 1991 May;34(5):505–514. doi: 10.1002/art.1780340502. [DOI] [PubMed] [Google Scholar]
- Arden N. K., Griffiths G. O., Hart D. J., Doyle D. V., Spector T. D. The association between osteoarthritis and osteoporotic fracture: the Chingford Study. Br J Rheumatol. 1996 Dec;35(12):1299–1304. doi: 10.1093/rheumatology/35.12.1299. [DOI] [PubMed] [Google Scholar]
- Arokoski J. P., Jurvelin J. S., Vätäinen U., Helminen H. J. Normal and pathological adaptations of articular cartilage to joint loading. Scand J Med Sci Sports. 2000 Aug;10(4):186–198. doi: 10.1034/j.1600-0838.2000.010004186.x. [DOI] [PubMed] [Google Scholar]
- Belmonte-Serrano M. A., Bloch D. A., Lane N. E., Michel B. E., Fries J. F. The relationship between spinal and peripheral osteoarthritis and bone density measurements. J Rheumatol. 1993 Jun;20(6):1005–1013. [PubMed] [Google Scholar]
- Brandt K. D., Braunstein E. M., Visco D. M., O'Connor B., Heck D., Albrecht M. Anterior (cranial) cruciate ligament transection in the dog: a bona fide model of osteoarthritis, not merely of cartilage injury and repair. J Rheumatol. 1991 Mar;18(3):436–446. [PubMed] [Google Scholar]
- Bruno R. J., Sauer P. A., Rosenberg A. G., Block J., Sumner D. R. The pattern of bone mineral density in the proximal femur and radiographic signs of early joint degeneration. J Rheumatol. 1999 Mar;26(3):636–640. [PubMed] [Google Scholar]
- Buckwalter J. A., Mankin H. J. Articular cartilage: degeneration and osteoarthritis, repair, regeneration, and transplantation. Instr Course Lect. 1998;47:487–504. [PubMed] [Google Scholar]
- Burger H., van Daele P. L., Odding E., Valkenburg H. A., Hofman A., Grobbee D. E., Schütte H. E., Birkenhäger J. C., Pols H. A. Association of radiographically evident osteoarthritis with higher bone mineral density and increased bone loss with age. The Rotterdam Study. Arthritis Rheum. 1996 Jan;39(1):81–86. doi: 10.1002/art.1780390111. [DOI] [PubMed] [Google Scholar]
- Burr D. B. The importance of subchondral bone in osteoarthrosis. Curr Opin Rheumatol. 1998 May;10(3):256–262. doi: 10.1097/00002281-199805000-00017. [DOI] [PubMed] [Google Scholar]
- Carlson C. S., Loeser R. F., Jayo M. J., Weaver D. S., Adams M. R., Jerome C. P. Osteoarthritis in cynomolgus macaques: a primate model of naturally occurring disease. J Orthop Res. 1994 May;12(3):331–339. doi: 10.1002/jor.1100120305. [DOI] [PubMed] [Google Scholar]
- Carlson C. S., Loeser R. F., Purser C. B., Gardin J. F., Jerome C. P. Osteoarthritis in cynomolgus macaques. III: Effects of age, gender, and subchondral bone thickness on the severity of disease. J Bone Miner Res. 1996 Sep;11(9):1209–1217. doi: 10.1002/jbmr.5650110904. [DOI] [PubMed] [Google Scholar]
- Carlsson A., Nilsson B. E., Westlin N. E. Bone mass in primary coxarthrosis. Acta Orthop Scand. 1979 Apr;50(2):187–189. doi: 10.3109/17453677908989755. [DOI] [PubMed] [Google Scholar]
- Cooper C., Cook P. L., Osmond C., Fisher L., Cawley M. I. Osteoarthritis of the hip and osteoporosis of the proximal femur. Ann Rheum Dis. 1991 Aug;50(8):540–542. doi: 10.1136/ard.50.8.540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper C., Poll V., McLaren M., Daunt S. O., Cawley M. I. Alterations in appendicular skeletal mass in patients with rheumatoid, psoriatic, and osteoarthropathy. Ann Rheum Dis. 1988 Jun;47(6):481–484. doi: 10.1136/ard.47.6.481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dedrick D. K., Goldstein S. A., Brandt K. D., O'Connor B. L., Goulet R. W., Albrecht M. A longitudinal study of subchondral plate and trabecular bone in cruciate-deficient dogs with osteoarthritis followed up for 54 months. Arthritis Rheum. 1993 Oct;36(10):1460–1467. doi: 10.1002/art.1780361019. [DOI] [PubMed] [Google Scholar]
- Dequeker J., Boonen S., Aerssens J., Westhovens R. Inverse relationship osteoarthritis-osteoporosis: what is the evidence? What are the consequences? Br J Rheumatol. 1996 Sep;35(9):813–818. doi: 10.1093/rheumatology/35.9.813. [DOI] [PubMed] [Google Scholar]
- Dequeker J. Inverse relationship of interface between osteoporosis and osteoarthritis. J Rheumatol. 1997 Apr;24(4):795–798. [PubMed] [Google Scholar]
- Dequeker J., Mohan S., Finkelman R. D., Aerssens J., Baylink D. J. Generalized osteoarthritis associated with increased insulin-like growth factor types I and II and transforming growth factor beta in cortical bone from the iliac crest. Possible mechanism of increased bone density and protection against osteoporosis. Arthritis Rheum. 1993 Dec;36(12):1702–1708. doi: 10.1002/art.1780361209. [DOI] [PubMed] [Google Scholar]
- Dequeker J., Mokassa L., Aerssens J. Bone density and osteoarthritis. J Rheumatol Suppl. 1995 Feb;43:98–100. [PubMed] [Google Scholar]
- Fazzalari N. L., Parkinson I. H. Femoral trabecular bone of osteoarthritic and normal subjects in an age and sex matched group. Osteoarthritis Cartilage. 1998 Nov;6(6):377–382. doi: 10.1053/joca.1998.0141. [DOI] [PubMed] [Google Scholar]
- Foss M. V., Byers P. D. Bone density, osteoarthrosis of the hip, and fracture of the upper end of the femur. Ann Rheum Dis. 1972 Jul;31(4):259–264. doi: 10.1136/ard.31.4.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girard M. S., Sartoris D. J., Moscona A. A., Ramos E. Measured femoral density by dual-energy X-ray absorptiometry as a function of rotation. Orthop Rev. 1994 Jan;23(1):38–40. [PubMed] [Google Scholar]
- Goker B., Sumner D. R., Hurwitz D. E., Block J. A. Bone mineral density varies as a function of the rate of joint space narrowing in the hip. J Rheumatol. 2000 Mar;27(3):735–738. [PubMed] [Google Scholar]
- Grynpas M. D., Alpert B., Katz I., Lieberman I., Pritzker K. P. Subchondral bone in osteoarthritis. Calcif Tissue Int. 1991 Jul;49(1):20–26. doi: 10.1007/BF02555898. [DOI] [PubMed] [Google Scholar]
- Hart D. J., Mootoosamy I., Doyle D. V., Spector T. D. The relationship between osteoarthritis and osteoporosis in the general population: the Chingford Study. Ann Rheum Dis. 1994 Mar;53(3):158–162. doi: 10.1136/ard.53.3.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hochberg M. C., Lethbridge-Cejku M., Scott W. W., Jr, Reichle R., Plato C. C., Tobin J. D. Upper extremity bone mass and osteoarthritis of the knees: data from the Baltimore Longitudinal Study of Aging. J Bone Miner Res. 1995 Mar;10(3):432–438. doi: 10.1002/jbmr.5650100314. [DOI] [PubMed] [Google Scholar]
- Hordon L. D., Stewart S. P., Troughton P. R., Wright V., Horsman A., Smith M. A. Primary generalized osteoarthritis and bone mass. Br J Rheumatol. 1993 Dec;32(12):1059–1061. doi: 10.1093/rheumatology/32.12.1059. [DOI] [PubMed] [Google Scholar]
- Huuskonen J., Väisänen S. B., Kröger H., Jurvelin C., Bouchard C., Alhava E., Rauramaa R. Determinants of bone mineral density in middle aged men: a population-based study. Osteoporos Int. 2000;11(8):702–708. doi: 10.1007/s001980070069. [DOI] [PubMed] [Google Scholar]
- Knight S. M., Ring E. F., Bhalla A. K. Bone mineral density and osteoarthritis. Ann Rheum Dis. 1992 Sep;51(9):1025–1026. doi: 10.1136/ard.51.9.1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kröger H., Kotaniemi A., Vainio P., Alhava E. Bone densitometry of the spine and femur in children by dual-energy x-ray absorptiometry. Bone Miner. 1992 Apr;17(1):75–85. doi: 10.1016/0169-6009(92)90712-m. [DOI] [PubMed] [Google Scholar]
- Kröger H., Vainio P., Nieminen J., Kotaniemi A. Comparison of different models for interpreting bone mineral density measurements using DXA and MRI technology. Bone. 1995 Aug;17(2):157–159. doi: 10.1016/s8756-3282(95)00162-x. [DOI] [PubMed] [Google Scholar]
- Lequesne M. G., Samson M. Indices of severity in osteoarthritis for weight bearing joints. J Rheumatol Suppl. 1991 Feb;27:16–18. [PubMed] [Google Scholar]
- Li B., Aspden R. M. Mechanical and material properties of the subchondral bone plate from the femoral head of patients with osteoarthritis or osteoporosis. Ann Rheum Dis. 1997 Apr;56(4):247–254. doi: 10.1136/ard.56.4.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li K. C., Higgs J., Aisen A. M., Buckwalter K. A., Martel W., McCune W. J. MRI in osteoarthritis of the hip: gradations of severity. Magn Reson Imaging. 1988 May-Jun;6(3):229–236. doi: 10.1016/0730-725x(88)90396-7. [DOI] [PubMed] [Google Scholar]
- Madsen O. R., Brot C., Petersen M. M., Sørensen O. H. Body composition and muscle strength in women scheduled for a knee or hip replacement. A comparative study of two groups of osteoarthritic women. Clin Rheumatol. 1997 Jan;16(1):39–44. doi: 10.1007/BF02238761. [DOI] [PubMed] [Google Scholar]
- Nevitt M. C., Lane N. E., Scott J. C., Hochberg M. C., Pressman A. R., Genant H. K., Cummings S. R. Radiographic osteoarthritis of the hip and bone mineral density. The Study of Osteoporotic Fractures Research Group. Arthritis Rheum. 1995 Jul;38(7):907–916. doi: 10.1002/art.1780380706. [DOI] [PubMed] [Google Scholar]
- Price T., Hesp R., Mitchell R. Bone density in generalized osteoarthritis. J Rheumatol. 1987 Jun;14(3):560–562. [PubMed] [Google Scholar]
- Radin E. L., Burr D. B., Caterson B., Fyhrie D., Brown T. D., Boyd R. D. Mechanical determinants of osteoarthrosis. Semin Arthritis Rheum. 1991 Dec;21(3 Suppl 2):12–21. doi: 10.1016/0049-0172(91)90036-y. [DOI] [PubMed] [Google Scholar]
- Reid D. M., Kennedy N. S., Smith M. A., Tothill P., Nuki G. Bone mass in nodal primary generalised osteoarthrosis. Ann Rheum Dis. 1984 Apr;43(2):240–242. doi: 10.1136/ard.43.2.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roh Y. S., Dequeker J., Mulier J. C. Bone mass in osteoarthrosis, measured in vivo by photon absorption. J Bone Joint Surg Am. 1974 Apr;56(3):587–591. [PubMed] [Google Scholar]
- Roh Y. S., Dequeker J., Mulier J. C. Cortical bone remodeling and bone mass in primary osteoarthrosis of the hip. Invest Radiol. 1973 Jul-Aug;8(4):351–354. [PubMed] [Google Scholar]
- Sambrook P., Naganathan V. What is the relationship between osteoarthritis and osteoporosis? Baillieres Clin Rheumatol. 1997 Nov;11(4):695–710. doi: 10.1016/s0950-3579(97)80005-2. [DOI] [PubMed] [Google Scholar]
- Solomon L., Schnitzler C. M., Browett J. P. Osteoarthritis of the hip: the patient behind the disease. Ann Rheum Dis. 1982 Apr;41(2):118–125. doi: 10.1136/ard.41.2.118. [DOI] [PMC free article] [PubMed] [Google Scholar]