Abstract
Objective: To study the pattern and cell type-specificity of collagenase 3, membrane-type 1 matrix metalloproteinase (MT1-MMP), and gelatinase A mRNA expression in the synovial membrane in rheumatoid arthritis (RA).
Methods: The mRNA expression of collagenase 3, MT1-MMP, and gelatinase A was characterised by northern blot analysis, reverse transcriptase-polymerase chain reaction, and in situ hybridisation. In situ hybridisation was performed in combination with the immunohistochemical detection of cell type-specific antigens.
Results: Synovial membrane specimens from 19 of 21 patients with RA expressing collagenase 3 mRNA were positive for MT1-MMP and gelatinase A mRNA. In control samples from patients without destructive inflammatory joint diseases collagenase 3 mRNA was not expressed and only in two of seven cases was a coexpression of MT1-MMP and gelatinase A mRNA detected. Fibroblast-like cells of the synovial membrane were found to be the predominant source of collagenase 3, MT1-MMP, and gelatinase A mRNA expression in lining and sublining layers as well as at the synovial membrane-cartilage interface. Additionally, the expression of MT1-MMP mRNA was detected in endothelial cells. Collagenase 3 mRNA expression was found in about 5% of CD68 positive macrophages.
Conclusions: Collagenase 3 mRNA is expressed simultaneously with MT1-MMP and gelatinase A mRNA in fibroblast-like cells of the synovial membrane in RA. These results suggest (a) a broad extracellular proteolytic potential of fibroblast-like cells and (b) an important role of cell surface associated procollagenase 3 activation by MT1-MMP and gelatinase A for cartilage degradation by invading fibroblast-like cells.
Full Text
The Full Text of this article is available as a PDF (277.7 KB).
Figure 1 .
In situ hybridisation of collagenase 3 and interstitial collagenase mRNA in the synovial membrane from patients with RA (NBT/BCIP colour reagent). Collagenase 3 mRNA was detected in lining and sublining layers (A), whereas the mRNA expression of interstitial collagenase was largely restricted to the synovial lining (B). These data were confirmed by analysing synovial membrane samples of six patients with RA. Scale bar 100 µm in A; 130 µm in B.
Figure 2 .
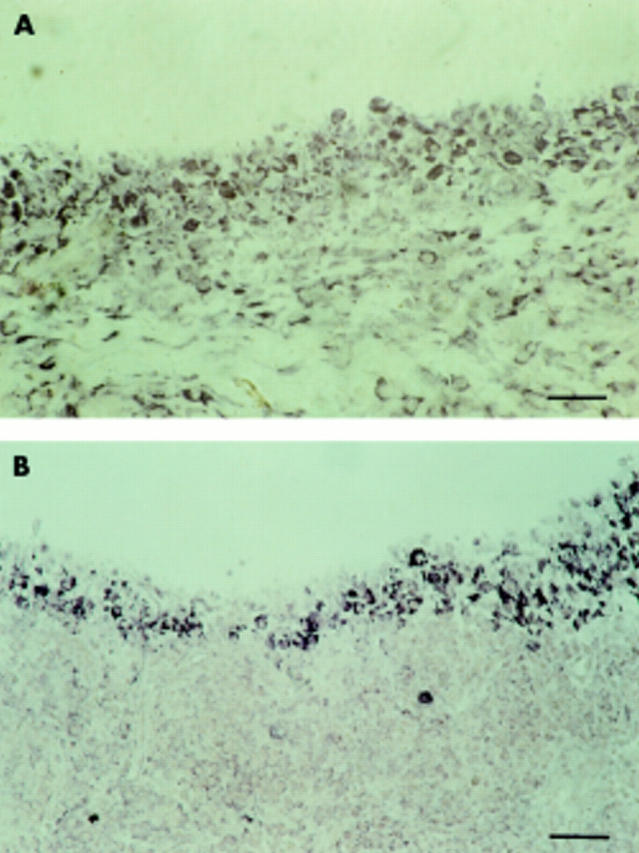
Cell type-specific mRNA expression of collagenase 3 in the synovial membrane in RA. Collagenase 3 mRNA was detected by in situ hybridisation (NBT/BCIP colour reagent; black) and expressed in fibroblast-like cells (arrowheads) of the synovial membrane which were immunohistochemically labelled with mAb D7-Fib (APAAP method; red) known to recognise fibroblasts (A). Lymphocytic infiltrates (L) in the synovial membrane were negative for collagenase 3 mRNA expression (B). Scale bar 80 µm in A; 130 µm in B.
Figure 3 .
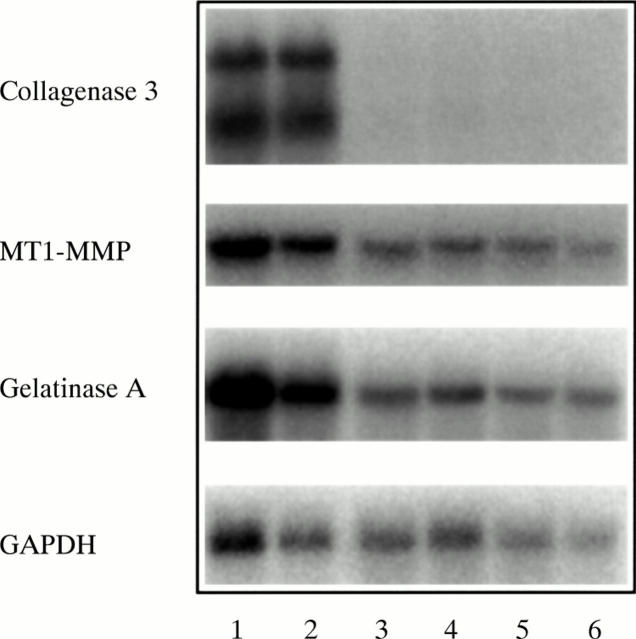
mRNA expression of collagenase 3, MT1-MMP, and gelatinase A in the synovial membrane in RA. Northern blot analysis was performed using total RNA from six separate synovial membrane specimens of patients with RA by successive hybridisation with radioactively labelled cDNA probes corresponding to collagenase 3, MT1-MMP, and gelatinase A, as indicated on the left. The mRNA expression levels of MT1-MMP and gelatinase A were only slightly raised in patients with RA expressing collagenase 3 mRNA in the synovial membrane (lanes 1 and 2) compared with patients without collagenase 3 mRNA expression (lane 3–6). GAPDH was used to check the loading of RNA.
Figure 4 .
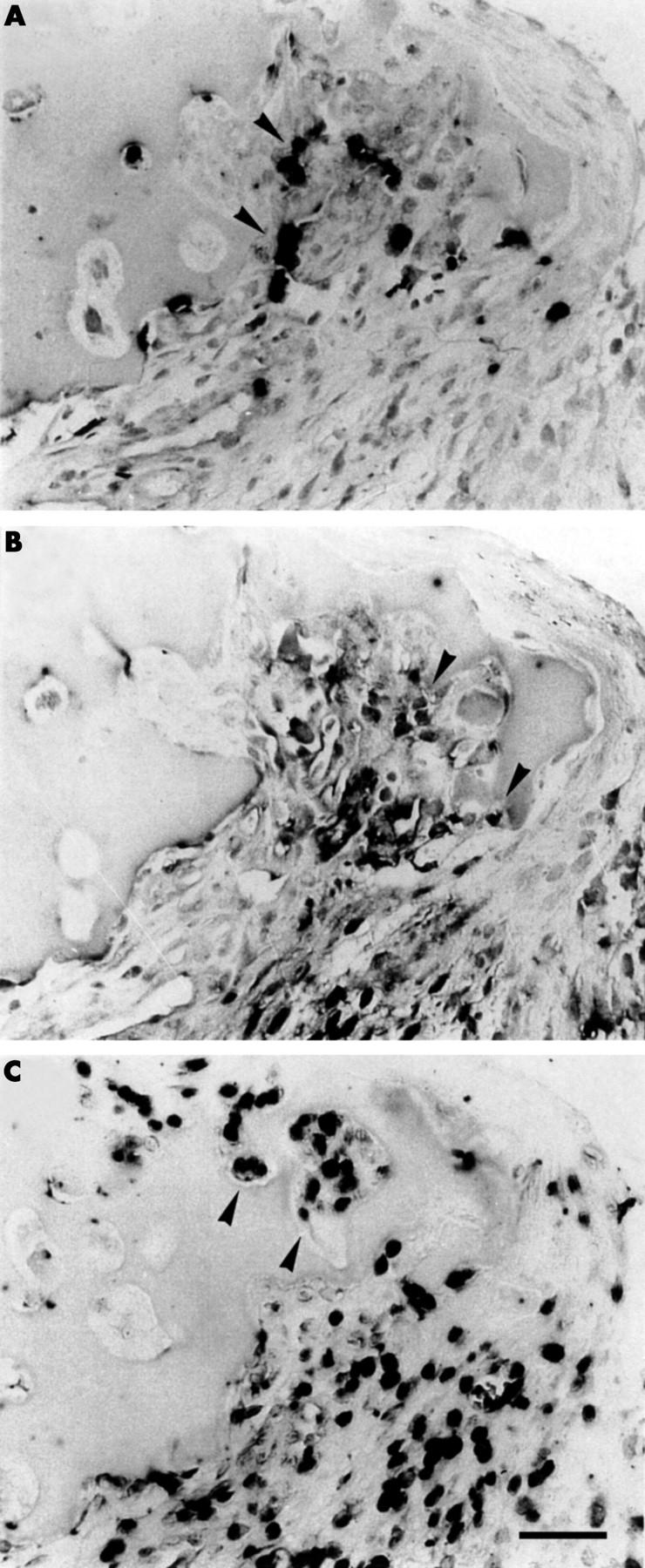
Localisation of collagenase 3, MT1-MMP, and gelatinase A mRNA expression at the synovial membrane-cartilage interface. Collagenase 3 (A), MT1-MMP (B), and gelatinase A mRNA (C) are colocalised in the synovial membrane adjacent to the cartilage (arrowheads), as shown for serial sections of the synovial membrane-cartilage interface of a patient with RA by in situ hybridisation (NBT/BCIP colour reagent; black). This observation was confirmed by analysing synovial membrane-cartilage samples of six patients with RA. Scale bar 100 µm.
Figure 5 .
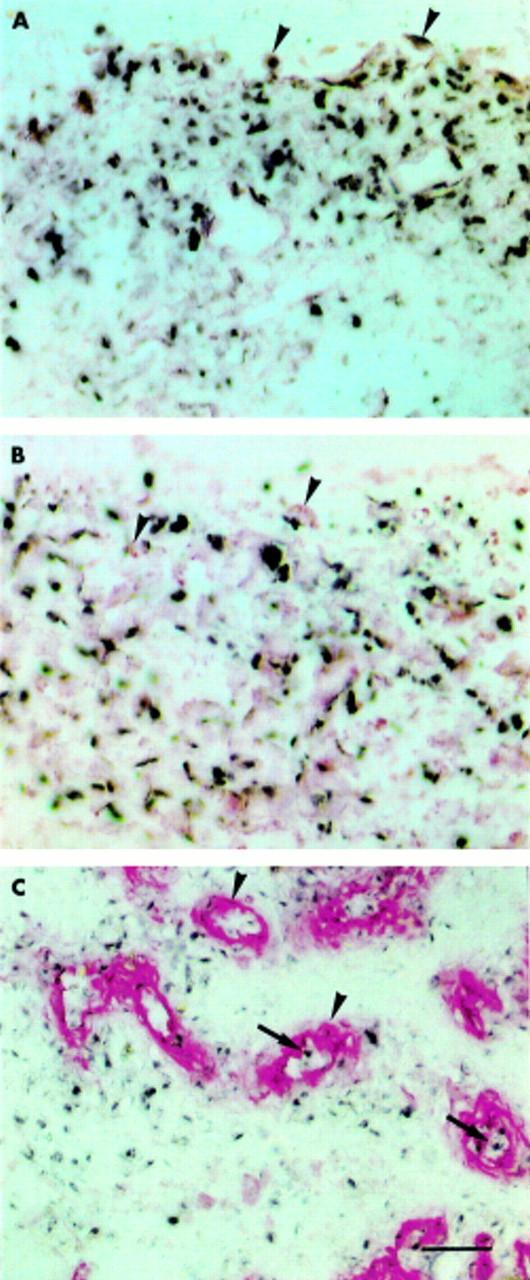
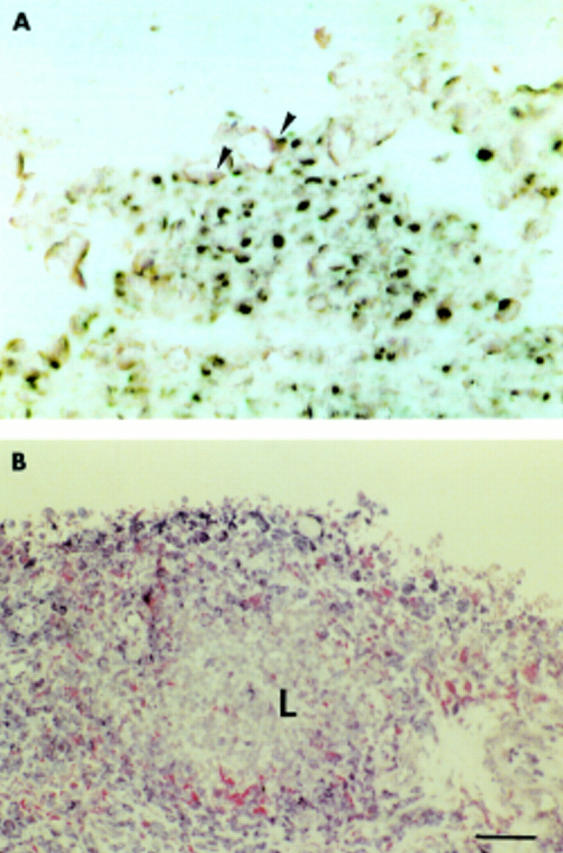
Cell type-specific mRNA expression of MT1-MMP and gelatinase A in the synovial membrane in RA. MT1-MMP and gelatinase A mRNA were detected by in situ hybridisation (NBT/BCIP colour reagent; black). Both MMPs are expressed in fibroblast-like cells (arrowheads) of the synovial membrane, which were immunohistochemically labelled with mAb D7-Fib (APAAP method; red) as shown in (A) and (B), respectively. MT1-MMP mRNA expression (black) was detected in endothelial cells (arrows) marked with an mAb against type IV collagen of the basal membrane (red, arrowheads) (C). Scale bar 80 µm in A and B; 200 µm C.
Figure 6 .
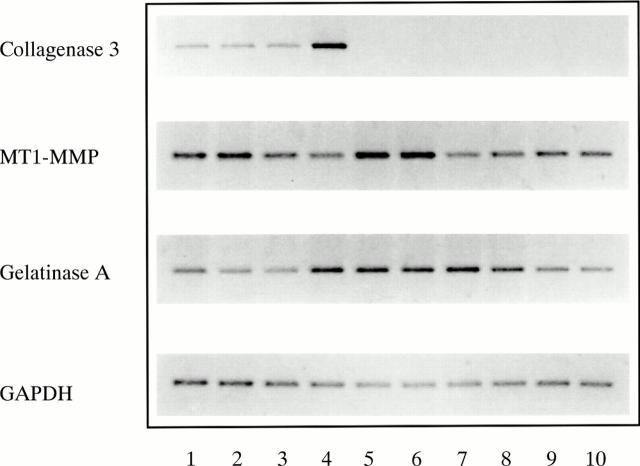
mRNA expression of collagenase 3, MT1-MMP, and gelatinase A in the synovial membrane in RA. RT-PCR was performed on total RNA from primary synovial fibroblast cultures of patients with RA using specific primers for collagenase 3, MT1-MMP, gelatinase A, and GAPDH as indicated on the left. All 10 fibroblast cell cultures expressed basal levels of MT1-MMP and gelatinase A mRNA, whereas basal collagenase 3 mRNA expression was detected only in 4/10 cultures.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnett F. C., Edworthy S. M., Bloch D. A., McShane D. J., Fries J. F., Cooper N. S., Healey L. A., Kaplan S. R., Liang M. H., Luthra H. S. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum. 1988 Mar;31(3):315–324. doi: 10.1002/art.1780310302. [DOI] [PubMed] [Google Scholar]
- Balbín M., Pendás A. M., Uría J. A., Jiménez M. G., Freije J. P., López-Otín C. Expression and regulation of collagenase-3 (MMP-13) in human malignant tumors. APMIS. 1999 Jan;107(1):45–53. doi: 10.1111/j.1699-0463.1999.tb01525.x. [DOI] [PubMed] [Google Scholar]
- Butler G. S., Butler M. J., Atkinson S. J., Will H., Tamura T., Schade van Westrum S., Crabbe T., Clements J., d'Ortho M. P., Murphy G. The TIMP2 membrane type 1 metalloproteinase "receptor" regulates the concentration and efficient activation of progelatinase A. A kinetic study. J Biol Chem. 1998 Jan 9;273(2):871–880. doi: 10.1074/jbc.273.2.871. [DOI] [PubMed] [Google Scholar]
- Gay S., Fine J. D. Characterization and isolation of poly- and monoclonal antibodies against collagen for use in immunohistochemistry. Methods Enzymol. 1987;145:148–167. doi: 10.1016/0076-6879(87)45007-6. [DOI] [PubMed] [Google Scholar]
- Haas T. L., Davis S. J., Madri J. A. Three-dimensional type I collagen lattices induce coordinate expression of matrix metalloproteinases MT1-MMP and MMP-2 in microvascular endothelial cells. J Biol Chem. 1998 Feb 6;273(6):3604–3610. doi: 10.1074/jbc.273.6.3604. [DOI] [PubMed] [Google Scholar]
- Hiraoka N., Allen E., Apel I. J., Gyetko M. R., Weiss S. J. Matrix metalloproteinases regulate neovascularization by acting as pericellular fibrinolysins. Cell. 1998 Oct 30;95(3):365–377. doi: 10.1016/s0092-8674(00)81768-7. [DOI] [PubMed] [Google Scholar]
- Hofmann U. B., Westphal J. R., Van Kraats A. A., Ruiter D. J., Van Muijen G. N. Expression of integrin alpha(v)beta(3) correlates with activation of membrane-type matrix metalloproteinase-1 (MT1-MMP) and matrix metalloproteinase-2 (MMP-2) in human melanoma cells in vitro and in vivo. Int J Cancer. 2000 Jul 1;87(1):12–19. doi: 10.1002/1097-0215(20000701)87:1<12::aid-ijc3>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- Imai K., Ohta S., Matsumoto T., Fujimoto N., Sato H., Seiki M., Okada Y. Expression of membrane-type 1 matrix metalloproteinase and activation of progelatinase A in human osteoarthritic cartilage. Am J Pathol. 1997 Jul;151(1):245–256. [PMC free article] [PubMed] [Google Scholar]
- Imanishi Y., Fujii M., Tokumaru Y., Tomita T., Kanke M., Kanzaki J., Kameyama K., Otani Y., Sato H. Clinical significance of expression of membrane type 1 matrix metalloproteinase and matrix metalloproteinase-2 in human head and neck squamous cell carcinoma. Hum Pathol. 2000 Aug;31(8):895–904. doi: 10.1053/hupa.2000.9756. [DOI] [PubMed] [Google Scholar]
- Johansson N., Airola K., Grénman R., Kariniemi A. L., Saarialho-Kere U., Kähäri V. M. Expression of collagenase-3 (matrix metalloproteinase-13) in squamous cell carcinomas of the head and neck. Am J Pathol. 1997 Aug;151(2):499–508. [PMC free article] [PubMed] [Google Scholar]
- Knäuper V., Cowell S., Smith B., López-Otin C., O'Shea M., Morris H., Zardi L., Murphy G. The role of the C-terminal domain of human collagenase-3 (MMP-13) in the activation of procollagenase-3, substrate specificity, and tissue inhibitor of metalloproteinase interaction. J Biol Chem. 1997 Mar 21;272(12):7608–7616. doi: 10.1074/jbc.272.12.7608. [DOI] [PubMed] [Google Scholar]
- Knäuper V., López-Otin C., Smith B., Knight G., Murphy G. Biochemical characterization of human collagenase-3. J Biol Chem. 1996 Jan 19;271(3):1544–1550. doi: 10.1074/jbc.271.3.1544. [DOI] [PubMed] [Google Scholar]
- Knäuper V., Will H., López-Otin C., Smith B., Atkinson S. J., Stanton H., Hembry R. M., Murphy G. Cellular mechanisms for human procollagenase-3 (MMP-13) activation. Evidence that MT1-MMP (MMP-14) and gelatinase a (MMP-2) are able to generate active enzyme. J Biol Chem. 1996 Jul 19;271(29):17124–17131. doi: 10.1074/jbc.271.29.17124. [DOI] [PubMed] [Google Scholar]
- Konttinen Y. T., Ainola M., Valleala H., Ma J., Ida H., Mandelin J., Kinne R. W., Santavirta S., Sorsa T., López-Otín C. Analysis of 16 different matrix metalloproteinases (MMP-1 to MMP-20) in the synovial membrane: different profiles in trauma and rheumatoid arthritis. Ann Rheum Dis. 1999 Nov;58(11):691–697. doi: 10.1136/ard.58.11.691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konttinen Y. T., Ceponis A., Takagi M., Ainola M., Sorsa T., Sutinen M., Salo T., Ma J., Santavirta S., Seiki M. New collagenolytic enzymes/cascade identified at the pannus-hard tissue junction in rheumatoid arthritis: destruction from above. Matrix Biol. 1998 Dec;17(8-9):585–601. doi: 10.1016/s0945-053x(98)90110-x. [DOI] [PubMed] [Google Scholar]
- Konttinen Y. T., Salo T., Hanemaaijer R., Valleala H., Sorsa T., Sutinen M., Ceponis A., Xu J. W., Santavirta S., Teronen O. Collagenase-3 (MMP-13) and its activators in rheumatoid arthritis: localization in the pannus-hard tissue junction and inhibition by alendronate. Matrix Biol. 1999 Aug;18(4):401–412. doi: 10.1016/s0945-053x(99)00030-x. [DOI] [PubMed] [Google Scholar]
- Kriegsmann J., Keyszer G., Geiler T., Gay R. E., Gay S. A new double labeling technique for combined in situ hybridization and immunohistochemical analysis. Lab Invest. 1994 Dec;71(6):911–917. [PubMed] [Google Scholar]
- Lindy O., Konttinen Y. T., Sorsa T., Ding Y., Santavirta S., Ceponis A., López-Otín C. Matrix metalloproteinase 13 (collagenase 3) in human rheumatoid synovium. Arthritis Rheum. 1997 Aug;40(8):1391–1399. doi: 10.1002/art.1780400806. [DOI] [PubMed] [Google Scholar]
- Mitchell P. G., Magna H. A., Reeves L. M., Lopresti-Morrow L. L., Yocum S. A., Rosner P. J., Geoghegan K. F., Hambor J. E. Cloning, expression, and type II collagenolytic activity of matrix metalloproteinase-13 from human osteoarthritic cartilage. J Clin Invest. 1996 Feb 1;97(3):761–768. doi: 10.1172/JCI118475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moldovan F., Pelletier J. P., Hambor J., Cloutier J. M., Martel-Pelletier J. Collagenase-3 (matrix metalloprotease 13) is preferentially localized in the deep layer of human arthritic cartilage in situ: in vitro mimicking effect by transforming growth factor beta. Arthritis Rheum. 1997 Sep;40(9):1653–1661. doi: 10.1002/art.1780400915. [DOI] [PubMed] [Google Scholar]
- Nagase H. Activation mechanisms of matrix metalloproteinases. Biol Chem. 1997 Mar-Apr;378(3-4):151–160. [PubMed] [Google Scholar]
- Pap T., Shigeyama Y., Kuchen S., Fernihough J. K., Simmen B., Gay R. E., Billingham M., Gay S. Differential expression pattern of membrane-type matrix metalloproteinases in rheumatoid arthritis. Arthritis Rheum. 2000 Jun;43(6):1226–1232. doi: 10.1002/1529-0131(200006)43:6<1226::AID-ANR5>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- Pendás A. M., Balbín M., Llano E., Jiménez M. G., López-Otín C. Structural analysis and promoter characterization of the human collagenase-3 gene (MMP13). Genomics. 1997 Mar 1;40(2):222–233. doi: 10.1006/geno.1996.4554. [DOI] [PubMed] [Google Scholar]
- Strongin A. Y., Collier I., Bannikov G., Marmer B. L., Grant G. A., Goldberg G. I. Mechanism of cell surface activation of 72-kDa type IV collagenase. Isolation of the activated form of the membrane metalloprotease. J Biol Chem. 1995 Mar 10;270(10):5331–5338. doi: 10.1074/jbc.270.10.5331. [DOI] [PubMed] [Google Scholar]
- Tardif G., Pelletier J. P., Dupuis M., Hambor J. E., Martel-Pelletier J. Cloning, sequencing and characterization of the 5'-flanking region of the human collagenase-3 gene. Biochem J. 1997 Apr 1;323(Pt 1):13–16. doi: 10.1042/bj3230013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tetlow L. C., Woolley D. E. Comparative immunolocalization studies of collagenase 1 and collagenase 3 production in the rheumatoid lesion, and by human chondrocytes and synoviocytes in vitro. Br J Rheumatol. 1998 Jan;37(1):64–70. doi: 10.1093/rheumatology/37.1.64. [DOI] [PubMed] [Google Scholar]
- Uría J. A., Jiménez M. G., Balbín M., Freije J. M., López-Otín C. Differential effects of transforming growth factor-beta on the expression of collagenase-1 and collagenase-3 in human fibroblasts. J Biol Chem. 1998 Apr 17;273(16):9769–9777. doi: 10.1074/jbc.273.16.9769. [DOI] [PubMed] [Google Scholar]
- Uría J. A., Ståhle-Bäckdahl M., Seiki M., Fueyo A., López-Otín C. Regulation of collagenase-3 expression in human breast carcinomas is mediated by stromal-epithelial cell interactions. Cancer Res. 1997 Nov 1;57(21):4882–4888. [PubMed] [Google Scholar]
- Vaalamo M., Mattila L., Johansson N., Kariniemi A. L., Karjalainen-Lindsberg M. L., Kähäri V. M., Saarialho-Kere U. Distinct populations of stromal cells express collagenase-3 (MMP-13) and collagenase-1 (MMP-1) in chronic ulcers but not in normally healing wounds. J Invest Dermatol. 1997 Jul;109(1):96–101. doi: 10.1111/1523-1747.ep12276722. [DOI] [PubMed] [Google Scholar]
- Werb Z. ECM and cell surface proteolysis: regulating cellular ecology. Cell. 1997 Nov 14;91(4):439–442. doi: 10.1016/s0092-8674(00)80429-8. [DOI] [PubMed] [Google Scholar]
- Wernicke D., Seyfert C., Hinzmann B., Gromnica-Ihle E. Cloning of collagenase 3 from the synovial membrane and its expression in rheumatoid arthritis and osteoarthritis. J Rheumatol. 1996 Apr;23(4):590–595. [PubMed] [Google Scholar]
- Westhoff C. S., Freudiger D., Petrow P., Seyfert C., Zacher J., Kriegsmann J., Pap T., Gay S., Stiehl P., Gromnica-Ihle E. Characterization of collagenase 3 (matrix metalloproteinase 13) messenger RNA expression in the synovial membrane and synovial fibroblasts of patients with rheumatoid arthritis. Arthritis Rheum. 1999 Jul;42(7):1517–1527. doi: 10.1002/1529-0131(199907)42:7<1517::AID-ANR27>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- Will H., Atkinson S. J., Butler G. S., Smith B., Murphy G. The soluble catalytic domain of membrane type 1 matrix metalloproteinase cleaves the propeptide of progelatinase A and initiates autoproteolytic activation. Regulation by TIMP-2 and TIMP-3. J Biol Chem. 1996 Jul 19;271(29):17119–17123. doi: 10.1074/jbc.271.29.17119. [DOI] [PubMed] [Google Scholar]
- Woessner J. F., Jr The family of matrix metalloproteinases. Ann N Y Acad Sci. 1994 Sep 6;732:11–21. doi: 10.1111/j.1749-6632.1994.tb24720.x. [DOI] [PubMed] [Google Scholar]
- Yamanaka H., Makino K., Takizawa M., Nakamura H., Fujimoto N., Moriya H., Nemori R., Sato H., Seiki M., Okada Y. Expression and tissue localization of membrane-types 1, 2, and 3 matrix metalloproteinases in rheumatoid synovium. Lab Invest. 2000 May;80(5):677–687. doi: 10.1038/labinvest.3780071. [DOI] [PubMed] [Google Scholar]