Abstract
Background: An abnormal distribution of subsets of γδ T cells, which are a component of the inflammatory infiltrate in arthritic synovium, has been demonstrated in the peripheral blood (PB) of patients with arthritis and neutropenia.
Objective: To evaluate whether the clinical manifestations of patients with arthritis and neutropenia are related to the specific γδ T cell subset predominant in the PB.
Methods: Flow cytometry of PB lymphocytes in six consecutive patients with chronic neutropenia and arthritis was performed. Variable (V) γ and δ gene families were analysed by polymerase chain reaction. cDNA was subjected to direct automated sequencing of T cell receptor (TCR) genes.
Results: Three patients had non-deforming and non-erosive rheumatoid factor (RF)+ polyarticular rheumatoid arthritis, RF+ oligoarticular arthritis, or RF- non-deforming oligoarticular psoriatic arthritis with persistent expansions of Vγ1+/Vδ2+, Vγ2+/Vδ2+, or Vγ1+/Vδ undetermined (2- 1-) T cells, respectively. The other three patients, without persistent expansion of γδ T cells, had either non-deforming and non-erosive oligo- or polyarthritis with a balanced distribution of several Vδ and Vγ genes, or severe erosive RF+ arthritis with deficiency of all but Vγ1+/Vδ1+ T cells.
Conclusions: γδ T cell lymphoproliferations in chronic neutropenia and arthritis use different Vγ and Vδ gene families, often forming T cell receptor (TCR) structures that are infrequent in normal adult PB. Arthritis with Vγ1+/Vδ2+, Vγ2+/Vδ2+, or Vγ1+/Vδ2-/Vδ1- γδ T cells in the PB is non-deforming and non-erosive, suggesting a protective effect of these cells, as opposed to a more pathogenic contribution of Vγ1+/Vδ1+ cells.
Full Text
The Full Text of this article is available as a PDF (168.8 KB).
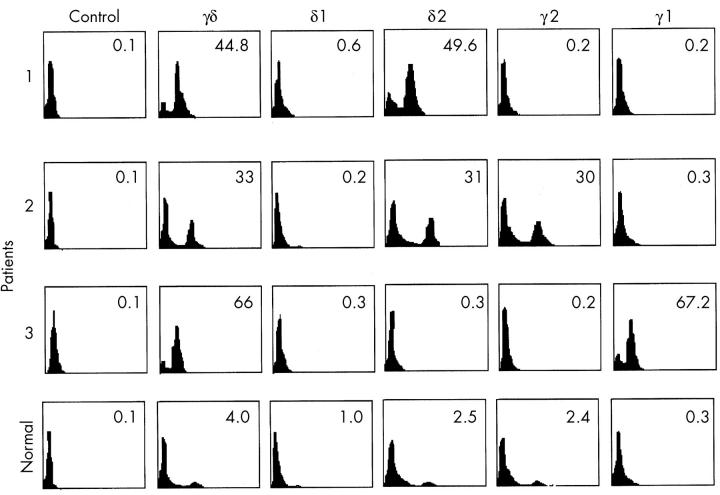
Figure 1 .
Analysis of T cell subsets in patient PBL. FACS analysis of patient PBL with mAb directed against the indicated antigens. Percentages of the indicated subsets are shown.
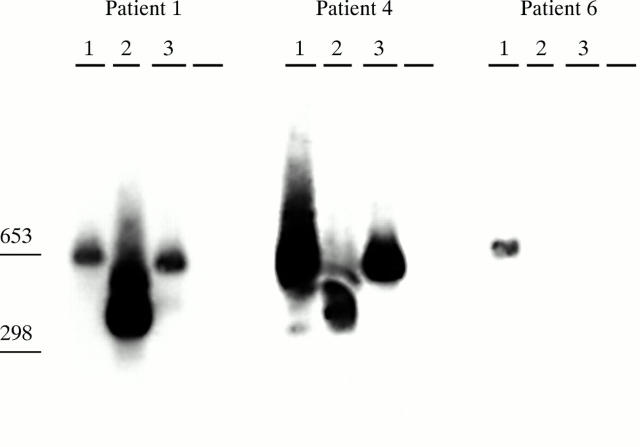
Figure 2 .
Vδ genes in patient PB. Southern blot representing DNA fragments amplified from PBL. PCR was employed using 5` Vδ gene family specific primers (Vδ1, Vδ2, and Vδ3 corresponding to lanes 1, 2, and 3) matched to Cδ gene specific primers.
Figure 3 .
(A) Vγ TCR gene amplification in clone B8. Each lane represents hybridisation with a Vγ family specific probe, after amplification with family specific Vγ primers as indicated and a 3` Cγ primer. (B) Nucleic acid and deduced amino acid sequence of patient 1 TCRδ genes. CδVδ2 amplified cDNA segments prepared from clone B8 and non-selected patient 1 PBL were sequenced in the 5` and 3` directions by the automated DNA sequencer ("Patients and methods"). The 5` and the 3` sequences were aligned and junctional sequences confirmed. The resulting matching sequences are aligned. The sequence has an in-frame Vδ2Dδ2Jδ1Cδ rearrangement and N region diversity. The deduced amino acid sequences are given below the nucleotide sequences.
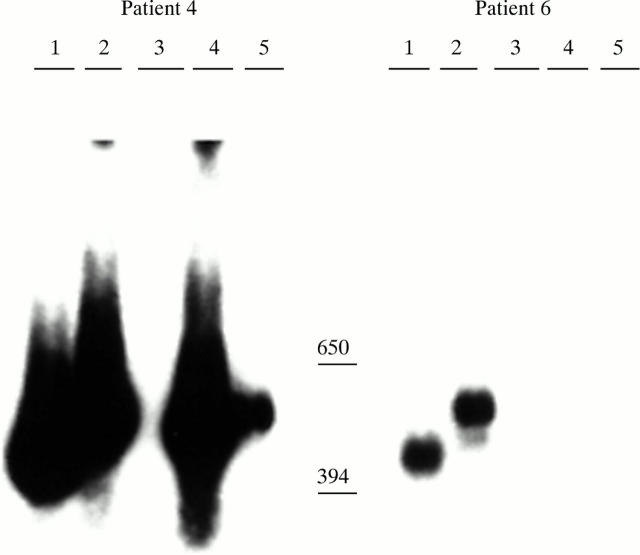
Figure 4 .
Vγ TCR gene amplification in PBL. Each lane represents hybridisation with a family specific Vγ probe, after amplification with a family specific 5` Vγ primer and a 3` Cγ primer. Five different Vγ primers (detecting Vγ1.2 in lane 1, Vγ1.2 and 1.4 in lane 2, Vγ1–1.8 in lane 3, Vγ1.8 in lane 4, and Vγ2 in lane 5) were used.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnett F. C., Edworthy S. M., Bloch D. A., McShane D. J., Fries J. F., Cooper N. S., Healey L. A., Kaplan S. R., Liang M. H., Luthra H. S. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum. 1988 Mar;31(3):315–324. doi: 10.1002/art.1780310302. [DOI] [PubMed] [Google Scholar]
- Bank I., Book M., Cohen L., Kneller A., Rosental E., Pras M., Bassat I. B., Ben-Nun A. Expansion of a unique subpopulation of cytotoxic T cells that express a C alpha V delta 1 T-cell receptor gene in a patient with severe persistent neutropenia. Blood. 1992 Dec 15;80(12):3157–3163. [PubMed] [Google Scholar]
- Bank I., DePinho R. A., Brenner M. B., Cassimeris J., Alt F. W., Chess L. A functional T3 molecule associated with a novel heterodimer on the surface of immature human thymocytes. Nature. 1986 Jul 10;322(6075):179–181. doi: 10.1038/322179a0. [DOI] [PubMed] [Google Scholar]
- Bank I., Tanay A., Migdal A., Book M., Livneh A. V gamma 9-V delta 2+ gamma delta T cells from a patient with Felty syndrome that exhibit aberrant response to triggering of the CD3 molecule can regulate immunoglobulin secretion by B cells. Clin Immunol Immunopathol. 1995 Feb;74(2):162–169. doi: 10.1006/clin.1995.1024. [DOI] [PubMed] [Google Scholar]
- Bowman S. J., Hall M. A., Panayi G. S., Lanchbury J. S. T cell receptor alpha-chain and beta-chain junctional region homology in clonal CD3+, CD8+ T lymphocyte expansions in Felty's syndrome. Arthritis Rheum. 1997 Apr;40(4):615–623. doi: 10.1002/art.1780400405. [DOI] [PubMed] [Google Scholar]
- Bukowski J. F., Morita C. T., Band H., Brenner M. B. Crucial role of TCR gamma chain junctional region in prenyl pyrophosphate antigen recognition by gamma delta T cells. J Immunol. 1998 Jul 1;161(1):286–293. [PubMed] [Google Scholar]
- Constant P., Davodeau F., Peyrat M. A., Poquet Y., Puzo G., Bonneville M., Fournié J. J. Stimulation of human gamma delta T cells by nonpeptidic mycobacterial ligands. Science. 1994 Apr 8;264(5156):267–270. doi: 10.1126/science.8146660. [DOI] [PubMed] [Google Scholar]
- Gladman D. D. Psoriatic arthritis. Rheum Dis Clin North Am. 1998 Nov;24(4):829-44, x. doi: 10.1016/s0889-857x(05)70044-2. [DOI] [PubMed] [Google Scholar]
- Haas W., Pereira P., Tonegawa S. Gamma/delta cells. Annu Rev Immunol. 1993;11:637–685. doi: 10.1146/annurev.iy.11.040193.003225. [DOI] [PubMed] [Google Scholar]
- Hodges E., Quin C., Farrell A. M., Christmas S., Sewell H. F., Doherty M., Powell R. J., Smith J. L. Arthropathy, leucopenia and recurrent infection associated with a TcR gamma delta population. Br J Rheumatol. 1995 Oct;34(10):978–983. doi: 10.1093/rheumatology/34.10.978. [DOI] [PubMed] [Google Scholar]
- Horiuchi T., Hirokawa M., Satoh K., Kitabayashi A., Muira A. B. Clonal expansion of gammadelta-T lymphocytes in an HTLV-I carrier, associated with chronic neutropenia and rheumatoid arthritis. Ann Hematol. 1999 Feb;78(2):101–104. doi: 10.1007/s002770050483. [DOI] [PubMed] [Google Scholar]
- Huber S. A., Graveline D., Newell M. K., Born W. K., O'Brien R. L. V gamma 1+ T cells suppress and V gamma 4+ T cells promote susceptibility to coxsackievirus B3-induced myocarditis in mice. J Immunol. 2000 Oct 15;165(8):4174–4181. doi: 10.4049/jimmunol.165.8.4174. [DOI] [PubMed] [Google Scholar]
- Ikebe H., Yamada H., Nomoto M., Takimoto H., Nakamura T., Sonoda K. H., Nomoto K. Persistent infection with Listeria monocytogenes in the kidney induces anti-inflammatory invariant fetal-type gammadelta T cells. Immunology. 2001 Jan;102(1):94–102. doi: 10.1046/j.1365-2567.2001.01149.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kabelitz D., Ackermann T., Hinz T., Davodeau F., Band H., Bonneville M., Janssen O., Arden B., Schondelmaier S. New monoclonal antibody (23D12) recognizing three different V gamma elements of the human gamma delta T cell receptor. 23D12+ cells comprise a major subpopulation of gamma delta T cells in postnatal thymus. J Immunol. 1994 Mar 15;152(6):3128–3136. [PubMed] [Google Scholar]
- Kaufmann S. H. gamma/delta and other unconventional T lymphocytes: what do they see and what do they do? Proc Natl Acad Sci U S A. 1996 Mar 19;93(6):2272–2279. doi: 10.1073/pnas.93.6.2272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keystone E. C., Rittershaus C., Wood N., Snow K. M., Flatow J., Purvis J. C., Poplonski L., Kung P. C. Elevation of a gamma delta T cell subset in peripheral blood and synovial fluid of patients with rheumatoid arthritis. Clin Exp Immunol. 1991 Apr;84(1):78–82. [PMC free article] [PubMed] [Google Scholar]
- Kuipers J. G., Jacobs R., Kemper A., Zeidler H., Schmidt R. E. TCR1+ large granular lymphocyte proliferation in rheumatoid arthritis. Rheumatol Int. 1994;14(4):163–168. doi: 10.1007/BF00579702. [DOI] [PubMed] [Google Scholar]
- Lahat N., Ben-Nun A., Cohen L., Kinarty A., Lerner A. T cell receptor repertoire in the peripheral blood and intestinal mucosa of coeliac patients. Clin Exp Immunol. 1995 Sep;101(3):422–427. doi: 10.1111/j.1365-2249.1995.tb03129.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lunardi C., Marguerie C., Walport M. J., So A. K. T gamma delta cells and their subsets in blood and synovial fluid from patients with rheumatoid arthritis. Br J Rheumatol. 1992 Aug;31(8):527–530. doi: 10.1093/rheumatology/31.8.527. [DOI] [PubMed] [Google Scholar]
- Morita C. T., Parker C. M., Brenner M. B., Band H. TCR usage and functional capabilities of human gamma delta T cells at birth. J Immunol. 1994 Nov 1;153(9):3979–3988. [PubMed] [Google Scholar]
- Mukasa A., Hiromatsu K., Matsuzaki G., O'Brien R., Born W., Nomoto K. Bacterial infection of the testis leading to autoaggressive immunity triggers apparently opposed responses of alpha beta and gamma delta T cells. J Immunol. 1995 Aug 15;155(4):2047–2056. [PubMed] [Google Scholar]
- Olive C., Gatenby P. A., Serjeantson S. W. Persistence of gamma/delta T cell oligoclonality in the peripheral blood of rheumatoid arthritis patients. Immunol Cell Biol. 1994 Feb;72(1):7–11. doi: 10.1038/icb.1994.2. [DOI] [PubMed] [Google Scholar]
- Peng S. L., Madaio M. P., Hayday A. C., Craft J. Propagation and regulation of systemic autoimmunity by gammadelta T cells. J Immunol. 1996 Dec 15;157(12):5689–5698. [PubMed] [Google Scholar]
- Pluschke G., Rüegg D., Hohlfeld R., Engel A. G. Autoaggressive myocytotoxic T lymphocytes expressing an unusual gamma/delta T cell receptor. J Exp Med. 1992 Dec 1;176(6):1785–1789. doi: 10.1084/jem.176.6.1785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenstein E. D., Kramer N. Felty's and pseudo-Felty's syndromes. Semin Arthritis Rheum. 1991 Dec;21(3):129–142. doi: 10.1016/0049-0172(91)90002-h. [DOI] [PubMed] [Google Scholar]
- Scott C. S., Richards S. J., Sivakumaran M., Steed A. J., Norfolk D. R., Milligan D. W., Short M. Persistent clonal expansions of CD3+TCR gamma delta+ and CD3+TCR alpha beta+CD4-CD8- lymphocytes associated with neutropenia. Leuk Lymphoma. 1994 Aug;14(5-6):429–440. doi: 10.3109/10428199409049700. [DOI] [PubMed] [Google Scholar]
- Sioud M., Førre O., Natvig J. B. T cell receptor delta diversity of freshly isolated T lymphocytes in rheumatoid synovitis. Eur J Immunol. 1991 Jan;21(1):239–241. doi: 10.1002/eji.1830210137. [DOI] [PubMed] [Google Scholar]
- Skelsey M. E., Mellon J., Niederkorn J. Y. Gamma delta T cells are needed for ocular immune privilege and corneal graft survival. J Immunol. 2001 Apr 1;166(7):4327–4333. doi: 10.4049/jimmunol.166.7.4327. [DOI] [PubMed] [Google Scholar]
- Smith M. D., Bröker B., Moretta L., Ciccone E., Grossi C. E., Edwards J. C., Yüksel F., Colaco B., Worman C., Mackenzie L. T gamma delta cells and their subsets in blood and synovial tissue from rheumatoid arthritis patients. Scand J Immunol. 1990 Dec;32(6):585–593. doi: 10.1111/j.1365-3083.1990.tb03200.x. [DOI] [PubMed] [Google Scholar]
- Tanaka Y., Morita C. T., Tanaka Y., Nieves E., Brenner M. B., Bloom B. R. Natural and synthetic non-peptide antigens recognized by human gamma delta T cells. Nature. 1995 May 11;375(6527):155–158. doi: 10.1038/375155a0. [DOI] [PubMed] [Google Scholar]
- Vincent M. S., Roessner K., Lynch D., Wilson D., Cooper S. M., Tschopp J., Sigal L. H., Budd R. C. Apoptosis of Fashigh CD4+ synovial T cells by borrelia-reactive Fas-ligand(high) gamma delta T cells in Lyme arthritis. J Exp Med. 1996 Dec 1;184(6):2109–2117. doi: 10.1084/jem.184.6.2109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wildner G., Hünig T., Thurau S. R. Orally induced, peptide-specific gamma/delta TCR+ cells suppress experimental autoimmune uveitis. Eur J Immunol. 1996 Sep;26(9):2140–2148. doi: 10.1002/eji.1830260927. [DOI] [PubMed] [Google Scholar]