Abstract
Objective: To characterise the expression of matrix metalloproteinases (MMPs) and tissue inhibitors of metalloproteinases (TIMPs) during degeneration of articular cartilage in a transgenic Del1 mouse model for osteoarthritis.
Methods: Northern analysis was used to measure mRNA levels of MMP-2, -3, -8, -9, -13, and -14, and TIMP-1, -2, and -3 in total RNA extracted from knee joints of transgenic Del1 mice, harbouring a 15 amino acid deletion in the triple helical domain of the α1(II) collagen chain, using their non-transgenic littermates as controls. Immunohistochemistry was used to study the presence of cleavage products (neoepitopes) of type II collagen, and the distribution of MMP-13 and TIMP-1 in degenerating cartilage.
Results: Each of the MMP and TIMP mRNAs analysed exhibited distinct expression patterns during development and osteoarthritic degeneration of the knee joint. The most striking change was up regulation of MMP-13 mRNA expression in the knee joints of Del1 mice at the onset of cartilage degeneration. However, the strongest immunostaining for MMP-13 and its inhibitor TIMP-1 was not seen in the degenerating articular cartilage but in synovial tissue, deep calcified cartilage, and subchondral bone. The localisation of type II collagen neoepitopes in chondrocytes and their pericellular matrix followed a similar pattern; they were not seen in cartilage fibrillations, but in adjacent unaffected cartilage.
Conclusion: The primary localisation of MMP-13 and TIMP-1 in hyperplastic synovial tissue, subchondral bone, and calcified cartilage suggests that up regulation of MMP-13 expression during early degeneration of articular cartilage is a secondary response to cartilage erosion. This interpretation is supported by the distribution of type II collagen neoepitopes. Synovial production of MMP-13 may be related to removal of tissue debris released from articular cartilage. In the deep calcified cartilage and adjacent subchondral bone, MMP-13 probably participates in tissue remodelling.
Full Text
The Full Text of this article is available as a PDF (250.2 KB).
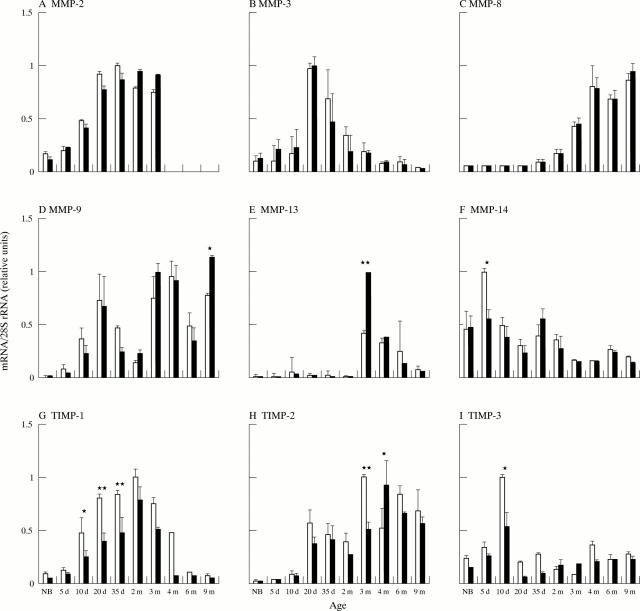
Figure 1 .
Compiled results of Northern analyses of knee joints for MMP-2 (A), MMP-3 (B), MMP-8 (C), MMP-9 (D), MMP-13 (E), MMP-14 (F), TIMP-1 (G), TIMP-2 (H), and TIMP-3 (I) mRNA levels. Total RNAs isolated from knee joints of transgenic Del1 mice and non-transgenic controls at different ages shown below the columns (NB, newborn; d, day; m, month) were analysed by Northern hybridisation. After hybridisation with cDNA probes which detected specific mRNAs of various MMPs and TIMP-1, -2, and -3, the hybridisation intensities were quantified by phosphor imaging and normalised per amount of 28S rRNA determined by hybridisation. The results are shown as relative units. Open columns denote control samples (n=6 at each time/age group), and black columns Del1 samples (n=6 at each time/age group). Statistical significance of the difference in mRNA levels between the control and Del1 mice is shown above the columns (Student's t test; *p<0.05; **p<0.01).
Figure 2 .
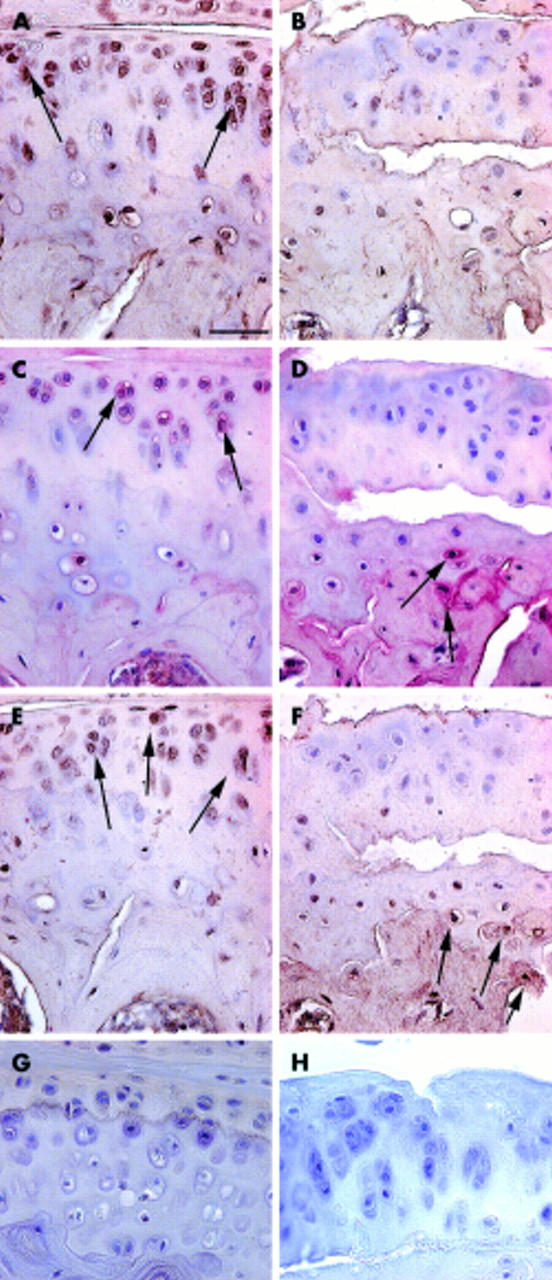
Immunolocalisation of type II collagen neoepitopes (A, B), MMP-13 (C, D), and TIMP-1 (E, F) during early stages of cartilage degeneration. Tissue sections from 3 month old Del1 (B, D, F) and control (A, C, E) mice. In control mice, type II collagen neoepitopes (A, arrows) and MMP-13 (C, arrows) were detected intracellularly and pericellularly in the uncalcified cartilage. In Del1 mice, MMP-13 was mainly localised in deep calcified cartilage and in subchondral bone (D, arrows). Also, TIMP-1 immunostaining was seen in the matrix of subchondral bone in Del1 mice (F). The distribution of TIMP-1 in control mice (E, arrows) resembled that of the type II collagen neoepitopes and MMP-13. Paraffin sections; avidin-biotin complex with horseradish peroxidase label and DAP detection (A, B, E, F) or alkaline phosphatase label and Fast Red detection (C, D), counterstaining with haematoxylin. Staining controls using normal rabbit serum (G) and without secondary antibody (H). The bar in panel A=25 µm.
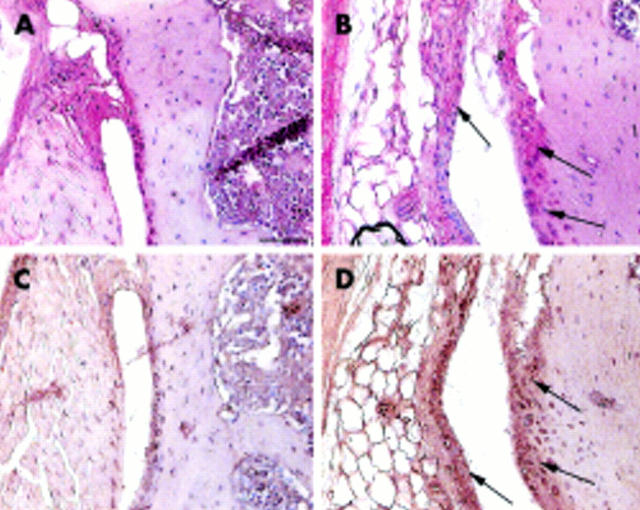
Figure 3 .
Appearance of the synovial membrane in control (A, C) and Del1 mice (B, D) at the age of 4 months. In Del1 mice, the synovial membrane of the knee joint was thickened and the immunostaining for MMP-13 (B, arrows) and TIMP-1 was increased (D, arrows) compared with the control knee joint (A, C, respectively). Paraffin sections; avidin-biotin complex with horseradish peroxidase label and DAP detection (C, D) or alkaline phosphatase label and Fast Red detection (A, B), counterstaining with haematoxylin. The bar in panel A=50 µm.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Apte S. S., Fukai N., Beier D. R., Olsen B. R. The matrix metalloproteinase-14 (MMP-14) gene is structurally distinct from other MMP genes and is co-expressed with the TIMP-2 gene during mouse embryogenesis. J Biol Chem. 1997 Oct 10;272(41):25511–25517. doi: 10.1074/jbc.272.41.25511. [DOI] [PubMed] [Google Scholar]
- Balbín M., Fueyo A., Knäuper V., López J. M., Alvarez J., Sánchez L. M., Quesada V., Bordallo J., Murphy G., López-Otín C. Identification and enzymatic characterization of two diverging murine counterparts of human interstitial collagenase (MMP-1) expressed at sites of embryo implantation. J Biol Chem. 2000 Dec 11;276(13):10253–10262. doi: 10.1074/jbc.M009586200. [DOI] [PubMed] [Google Scholar]
- Balbín M., Fueyo A., Knäuper V., Pendás A. M., López J. M., Jiménez M. G., Murphy G., López-Otín C. Collagenase 2 (MMP-8) expression in murine tissue-remodeling processes. Analysis of its potential role in postpartum involution of the uterus. J Biol Chem. 1998 Sep 11;273(37):23959–23968. doi: 10.1074/jbc.273.37.23959. [DOI] [PubMed] [Google Scholar]
- Billinghurst R. C., Dahlberg L., Ionescu M., Reiner A., Bourne R., Rorabeck C., Mitchell P., Hambor J., Diekmann O., Tschesche H. Enhanced cleavage of type II collagen by collagenases in osteoarthritic articular cartilage. J Clin Invest. 1997 Apr 1;99(7):1534–1545. doi: 10.1172/JCI119316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burr D. B. The importance of subchondral bone in osteoarthrosis. Curr Opin Rheumatol. 1998 May;10(3):256–262. doi: 10.1097/00002281-199805000-00017. [DOI] [PubMed] [Google Scholar]
- Büttner F. H., Chubinskaya S., Margerie D., Huch K., Flechtenmacher J., Cole A. A., Kuettner K. E., Bartnik E. Expression of membrane type 1 matrix metalloproteinase in human articular cartilage. Arthritis Rheum. 1997 Apr;40(4):704–709. doi: 10.1002/art.1780400415. [DOI] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Cole A. A., Chubinskaya S., Schumacher B., Huch K., Szabo G., Yao J., Mikecz K., Hasty K. A., Kuettner K. E. Chondrocyte matrix metalloproteinase-8. Human articular chondrocytes express neutrophil collagenase. J Biol Chem. 1996 May 3;271(18):11023–11026. doi: 10.1074/jbc.271.18.11023. [DOI] [PubMed] [Google Scholar]
- Dean D. D., Azzo W., Martel-Pelletier J., Pelletier J. P., Woessner J. F., Jr Levels of metalloproteases and tissue inhibitor of metalloproteases in human osteoarthritic cartilage. J Rheumatol. 1987 May;14(Spec No):43–44. [PubMed] [Google Scholar]
- Dean D. D., Martel-Pelletier J., Pelletier J. P., Howell D. S., Woessner J. F., Jr Evidence for metalloproteinase and metalloproteinase inhibitor imbalance in human osteoarthritic cartilage. J Clin Invest. 1989 Aug;84(2):678–685. doi: 10.1172/JCI114215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eerola I., Salminen H., Lammi P., Lammi M., von der Mark K., Vuorio E., Sämänen A. M. Type X collagen, a natural component of mouse articular cartilage: association with growth, aging, and osteoarthritis. Arthritis Rheum. 1998 Jul;41(7):1287–1295. doi: 10.1002/1529-0131(199807)41:7<1287::AID-ART20>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- Freemont A. J., Byers R. J., Taiwo Y. O., Hoyland J. A. In situ zymographic localisation of type II collagen degrading activity in osteoarthritic human articular cartilage. Ann Rheum Dis. 1999 Jun;58(6):357–365. doi: 10.1136/ard.58.6.357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freemont A. J., Hampson V., Tilman R., Goupille P., Taiwo Y., Hoyland J. A. Gene expression of matrix metalloproteinases 1, 3, and 9 by chondrocytes in osteoarthritic human knee articular cartilage is zone and grade specific. Ann Rheum Dis. 1997 Sep;56(9):542–549. doi: 10.1136/ard.56.9.542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamerman D. The biology of osteoarthritis. N Engl J Med. 1989 May 18;320(20):1322–1330. doi: 10.1056/NEJM198905183202006. [DOI] [PubMed] [Google Scholar]
- Hasty K. A., Jeffrey J. J., Hibbs M. S., Welgus H. G. The collagen substrate specificity of human neutrophil collagenase. J Biol Chem. 1987 Jul 25;262(21):10048–10052. [PubMed] [Google Scholar]
- Hembry R. M., Bagga M. R., Reynolds J. J., Hamblen D. L. Immunolocalisation studies on six matrix metalloproteinases and their inhibitors, TIMP-1 and TIMP-2, in synovia from patients with osteo- and rheumatoid arthritis. Ann Rheum Dis. 1995 Jan;54(1):25–32. doi: 10.1136/ard.54.1.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henriet P., Rousseau G. G., Eeckhout Y. Cloning and sequencing of mouse collagenase cDNA. Divergence of mouse and rat collagenases from the other mammalian collagenases. FEBS Lett. 1992 Sep 28;310(2):175–178. doi: 10.1016/0014-5793(92)81323-e. [DOI] [PubMed] [Google Scholar]
- Huebner J. L., Otterness I. G., Freund E. M., Caterson B., Kraus V. B. Collagenase 1 and collagenase 3 expression in a guinea pig model of osteoarthritis. Arthritis Rheum. 1998 May;41(5):877–890. doi: 10.1002/1529-0131(199805)41:5<877::AID-ART16>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- Huhtala P., Eddy R. L., Fan Y. S., Byers M. G., Shows T. B., Tryggvason K. Completion of the primary structure of the human type IV collagenase preproenzyme and assignment of the gene (CLG4) to the q21 region of chromosome 16. Genomics. 1990 Mar;6(3):554–559. doi: 10.1016/0888-7543(90)90486-e. [DOI] [PubMed] [Google Scholar]
- Joronen K., Salminen H., Glumoff V., Savontaus M., Vuorio E. Temporospatial expression of tissue inhibitors of matrix metalloproteinases-1, -2 and -3 during development, growth and aging of the mouse skeleton. Histochem Cell Biol. 2000 Aug;114(2):157–165. doi: 10.1007/s004180000177. [DOI] [PubMed] [Google Scholar]
- Keyszer G., Redlich A., Häupl T., Zacher J., Sparmann M., Engethüm U., Gay S., Burmester G. R. Differential expression of cathepsins B and L compared with matrix metalloproteinases and their respective inhibitors in rheumatoid arthritis and osteoarthritis: a parallel investigation by semiquantitative reverse transcriptase-polymerase chain reaction and immunohistochemistry. Arthritis Rheum. 1998 Aug;41(8):1378–1387. doi: 10.1002/1529-0131(199808)41:8<1378::AID-ART6>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- Knäuper V., López-Otin C., Smith B., Knight G., Murphy G. Biochemical characterization of human collagenase-3. J Biol Chem. 1996 Jan 19;271(3):1544–1550. doi: 10.1074/jbc.271.3.1544. [DOI] [PubMed] [Google Scholar]
- Kähäri V. M., Saarialho-Kere U. Matrix metalloproteinases and their inhibitors in tumour growth and invasion. Ann Med. 1999 Feb;31(1):34–45. doi: 10.3109/07853899909019260. [DOI] [PubMed] [Google Scholar]
- Lindy O., Konttinen Y. T., Sorsa T., Ding Y., Santavirta S., Ceponis A., López-Otín C. Matrix metalloproteinase 13 (collagenase 3) in human rheumatoid synovium. Arthritis Rheum. 1997 Aug;40(8):1391–1399. doi: 10.1002/art.1780400806. [DOI] [PubMed] [Google Scholar]
- Lohmander L. S., Lark M. W., Dahlberg L., Walakovits L. A., Roos H. Cartilage matrix metabolism in osteoarthritis: markers in synovial fluid, serum, and urine. Clin Biochem. 1992 Jun;25(3):167–174. doi: 10.1016/0009-9120(92)90250-v. [DOI] [PubMed] [Google Scholar]
- Manicourt D. H., Fujimoto N., Obata K., Thonar E. J. Serum levels of collagenase, stromelysin-1, and TIMP-1. Age- and sex-related differences in normal subjects and relationship to the extent of joint involvement and serum levels of antigenic keratan sulfate in patients with osteoarthritis. Arthritis Rheum. 1994 Dec;37(12):1774–1783. doi: 10.1002/art.1780371211. [DOI] [PubMed] [Google Scholar]
- Martel-Pelletier J., McCollum R., Fujimoto N., Obata K., Cloutier J. M., Pelletier J. P. Excess of metalloproteases over tissue inhibitor of metalloprotease may contribute to cartilage degradation in osteoarthritis and rheumatoid arthritis. Lab Invest. 1994 Jun;70(6):807–815. [PubMed] [Google Scholar]
- McDonnell S. E., Kerr L. D., Matrisian L. M. Epidermal growth factor stimulation of stromelysin mRNA in rat fibroblasts requires induction of proto-oncogenes c-fos and c-jun and activation of protein kinase C. Mol Cell Biol. 1990 Aug;10(8):4284–4293. doi: 10.1128/mcb.10.8.4284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metsäranta M., Garofalo S., Decker G., Rintala M., de Crombrugghe B., Vuorio E. Chondrodysplasia in transgenic mice harboring a 15-amino acid deletion in the triple helical domain of pro alpha 1(II) collagen chain. J Cell Biol. 1992 Jul;118(1):203–212. doi: 10.1083/jcb.118.1.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metsäranta M., Kujala U. M., Pelliniemi L., Osterman H., Aho H., Vuorio E. Evidence for insufficient chondrocytic differentiation during repair of full-thickness defects of articular cartilage. Matrix Biol. 1996 Apr;15(1):39–47. doi: 10.1016/s0945-053x(96)90125-0. [DOI] [PubMed] [Google Scholar]
- Mohtai M., Smith R. L., Schurman D. J., Tsuji Y., Torti F. M., Hutchinson N. I., Stetler-Stevenson W. G., Goldberg G. I. Expression of 92-kD type IV collagenase/gelatinase (gelatinase B) in osteoarthritic cartilage and its induction in normal human articular cartilage by interleukin 1. J Clin Invest. 1993 Jul;92(1):179–185. doi: 10.1172/JCI116547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moldovan F., Pelletier J. P., Hambor J., Cloutier J. M., Martel-Pelletier J. Collagenase-3 (matrix metalloprotease 13) is preferentially localized in the deep layer of human arthritic cartilage in situ: in vitro mimicking effect by transforming growth factor beta. Arthritis Rheum. 1997 Sep;40(9):1653–1661. doi: 10.1002/art.1780400915. [DOI] [PubMed] [Google Scholar]
- Mudgett J. S., Hutchinson N. I., Chartrain N. A., Forsyth A. J., McDonnell J., Singer I. I., Bayne E. K., Flanagan J., Kawka D., Shen C. F. Susceptibility of stromelysin 1-deficient mice to collagen-induced arthritis and cartilage destruction. Arthritis Rheum. 1998 Jan;41(1):110–121. doi: 10.1002/1529-0131(199801)41:1<110::AID-ART14>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- Naito K., Takahashi M., Kushida K., Suzuki M., Ohishi T., Miura M., Inoue T., Nagano A. Measurement of matrix metalloproteinases (MMPs) and tissue inhibitor of metalloproteinases-1 (TIMP-1) in patients with knee osteoarthritis: comparison with generalized osteoarthritis. Rheumatology (Oxford) 1999 Jun;38(6):510–515. doi: 10.1093/rheumatology/38.6.510. [DOI] [PubMed] [Google Scholar]
- Newman A. P. Articular cartilage repair. Am J Sports Med. 1998 Mar-Apr;26(2):309–324. doi: 10.1177/03635465980260022701. [DOI] [PubMed] [Google Scholar]
- Reponen P., Sahlberg C., Munaut C., Thesleff I., Tryggvason K. High expression of 92-kD type IV collagenase (gelatinase B) in the osteoclast lineage during mouse development. J Cell Biol. 1994 Mar;124(6):1091–1102. doi: 10.1083/jcb.124.6.1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salminen H., Perälä M., Lorenzo P., Saxne T., Heinegård D., Sämänen A. M., Vuorio E. Up-regulation of cartilage oligomeric matrix protein at the onset of articular cartilage degeneration in a transgenic mouse model of osteoarthritis. Arthritis Rheum. 2000 Aug;43(8):1742–1748. doi: 10.1002/1529-0131(200008)43:8<1742::AID-ANR10>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- Salminen H., Vuorio E., Sämänen A. M. Expression of Sox9 and type IIA procollagen during attempted repair of articular cartilage damage in a transgenic mouse model of osteoarthritis. Arthritis Rheum. 2001 Apr;44(4):947–955. doi: 10.1002/1529-0131(200104)44:4<947::AID-ANR152>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- Shlopov B. V., Lie W. R., Mainardi C. L., Cole A. A., Chubinskaya S., Hasty K. A. Osteoarthritic lesions: involvement of three different collagenases. Arthritis Rheum. 1997 Nov;40(11):2065–2074. doi: 10.1002/art.1780401120. [DOI] [PubMed] [Google Scholar]
- Smith M. D., Triantafillou S., Parker A., Youssef P. P., Coleman M. Synovial membrane inflammation and cytokine production in patients with early osteoarthritis. J Rheumatol. 1997 Feb;24(2):365–371. [PubMed] [Google Scholar]
- Sämänen A. K., Salminen H. J., Dean P. B., De Crombrugghe B., Vuorio E. I., Metsäranta M. P. Osteoarthritis-like lesions in transgenic mice harboring a small deletion mutation in type II collagen gene. Osteoarthritis Cartilage. 2000 Jul;8(4):248–257. doi: 10.1053/joca.2000.0298. [DOI] [PubMed] [Google Scholar]
- Tsuchiya K., Maloney W. J., Vu T., Hoffman A. R., Schurman D. J., Smith R. L. RT-PCR analysis of MMP-9 expression in human articular cartilage chondrocytes and synovial fluid cells. Biotech Histochem. 1996 Jul;71(4):208–213. doi: 10.3109/10520299609117161. [DOI] [PubMed] [Google Scholar]
- Vankemmelbeke M., Dekeyser P. M., Hollander A. P., Buttle D. J., Demeester J. Characterization of helical cleavages in type II collagen generated by matrixins. Biochem J. 1998 Mar 1;330(Pt 2):633–640. doi: 10.1042/bj3300633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilhelmi G., Faust R. Suitability of the C57 black mouse as an experimental animal for the study of skeletal changes due to ageing, with special reference to osteo-arthrosis and its response to tribenoside. Pharmacology. 1976;14(4):289–296. doi: 10.1159/000136607. [DOI] [PubMed] [Google Scholar]
- Yamagiwa H., Tokunaga K., Hayami T., Hatano H., Uchida M., Endo N., Takahashi H. E. Expression of metalloproteinase-13 (Collagenase-3) is induced during fracture healing in mice. Bone. 1999 Aug;25(2):197–203. doi: 10.1016/s8756-3282(99)00157-x. [DOI] [PubMed] [Google Scholar]
- Zafarullah M., Pelletier J. P., Cloutier J. M., Martel-Pelletier J. Elevated metalloproteinase and tissue inhibitor of metalloproteinase mRNA in human osteoarthritic synovia. J Rheumatol. 1993 Apr;20(4):693–697. [PubMed] [Google Scholar]