Abstract
Objectives: To investigate the effects of voluntary running on the incidence and severity of osteoarthritis (OA) and associated changes in cartilage matrix and subchondral bone in a transgenic Del1 mouse model for OA.
Methods: Del1 mice and their non-transgenic littermate controls were housed from the age of 5–6 weeks to 15 months in individual cages with running wheels. The running activity of each mouse was monitored for the entire 12 month period. Additional Del1 and control mice were housed in individual cages without running wheels. At the end of the experiment the severity of OA was evaluated by light microscopy, and the articular cartilage matrix changes by digital densitometry and quantitative polarised light microscopy.
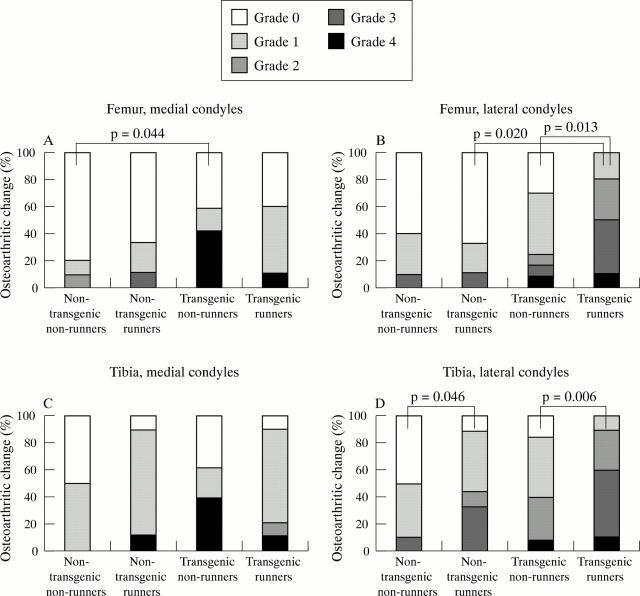
Results: Lifelong voluntary running increased the incidence and severity of OA significantly in Del1 mice (transgenic runners), and slightly also in non-transgenic runners. Severe OA changes increased from 39% in transgenic non-runners to 90% in transgenic runners (p=0.006) in lateral tibial condyles, and from 24% to 80% (p=0.013) in lateral femoral condyles, respectively. The proteoglycan content of articular cartilage was reduced in transgenic runners in comparison with transgenic non-runners (p=0.0167), but a similar effect was not seen in non-transgenic runners compared with non-transgenic non-runners. No attributable differences were seen in the collagen network of articular cartilage or in the subchondral bone between any of the groups.
Conclusion: The Del1 mutation has earlier been shown to disturb the assembly of the cartilage collagen network and thereby increase the incidence and severity of OA with age. In this study, voluntary running was shown to increase further cartilage damage in the lateral compartments of the knee. This suggests that articular cartilage in Del1 mice is less resistant to physical loading than in control mice. Despite severe OA lesions in the knee joint at the age of 15 months, Del1 mice continued to run voluntarily 2–3 km every night.
Full Text
The Full Text of this article is available as a PDF (194.8 KB).
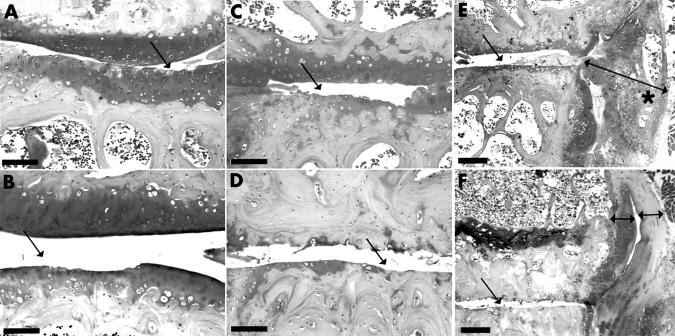
Figure 1 .
Scoring of OA changes from the frontal tibiofemoral joint sections of the lateral joint compartment. In each figure the arrow points to the grade in question. (A) Grade 1 OA, superficial fibrillation, cell loss from the superficial zone, and striation of the cartilage. (B) Grade 2 OA, deeper defects extending into the uncalcified cartilage. (C) Grade 3 OA, defects extending into the calcified cartilage. (D) Grade 4 OA, lesions extending in the subchondral bone which exhibits sclerosis. (E) Synovial and joint capsule reaction (line with two arrowheads) and grade 3 OA. Asterisk indicates the presence of calcified, bone-like tissue. (F) Grade 4 OA and hypertrophied synovium and joint capsule (lines with two arrowheads). Scale bar = 100 µm. Safranin O staining.
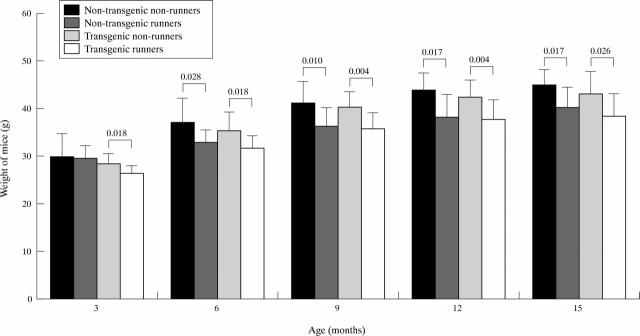
Figure 2 .
Weight development of the mice (mean (SD)). Mann-Whitney's U test.
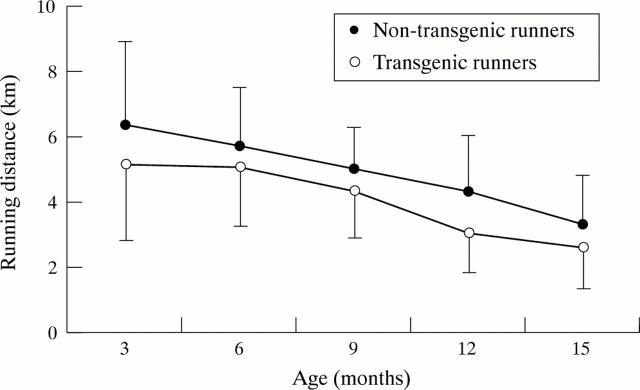
Figure 3 .
Daily running distances of the mice (mean (SD)).
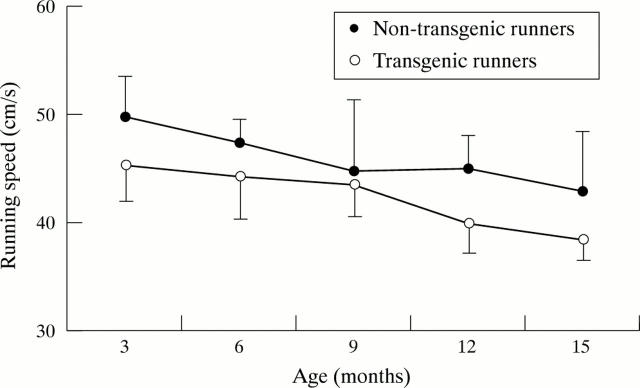
Figure 4 .
Average running speed of the mice (mean (SD)).
Figure 5 .
Osteoarthritic changes in the knee joints of mice at the age of 15 months. Mann-Whitney's U test.
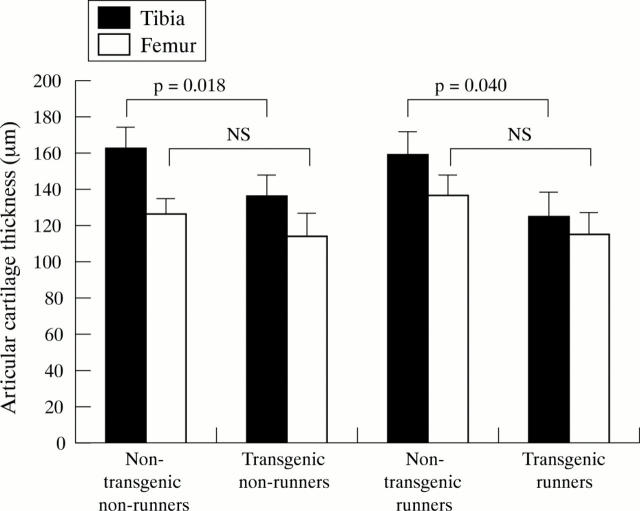
Figure 6 .
Articular cartilage thickness (mean (SEM)) in medial tibial and femoral condyles at the age of 15 months. NS, non significant, Mann-Whitney's U test.
Figure 7 .
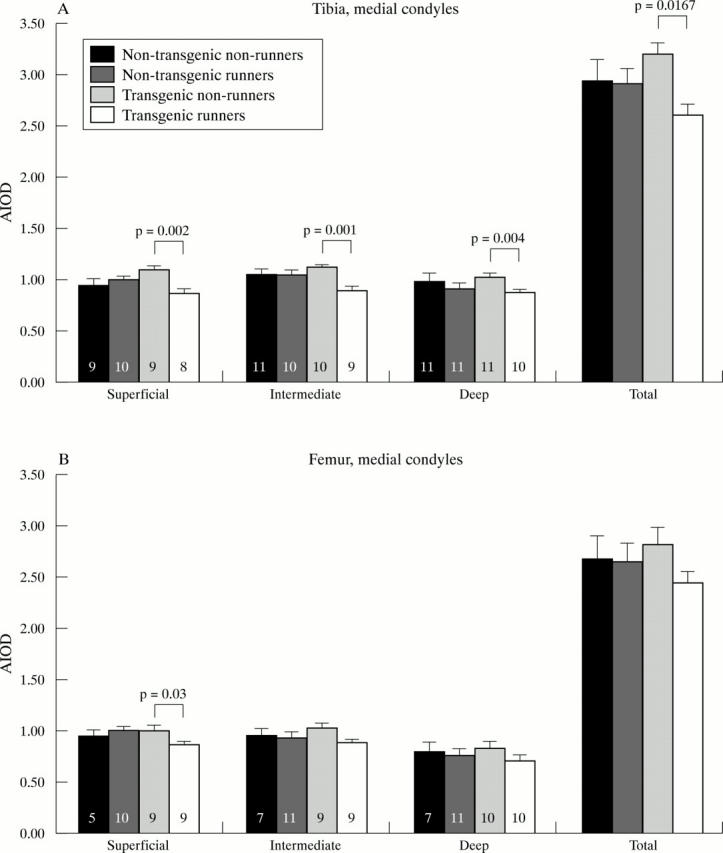
Safranin O stain absorbances in the superficial, intermediate, and deep zones of medial tibial (A) and femoral (B) cartilage condyles. "Total" indicates the cumulative absorbance in all zones. The number of mice analysed in each group and each zone is given as a number in the bars. Absorbance is given in AIOD (SEM). Mann-Whitney's U test.
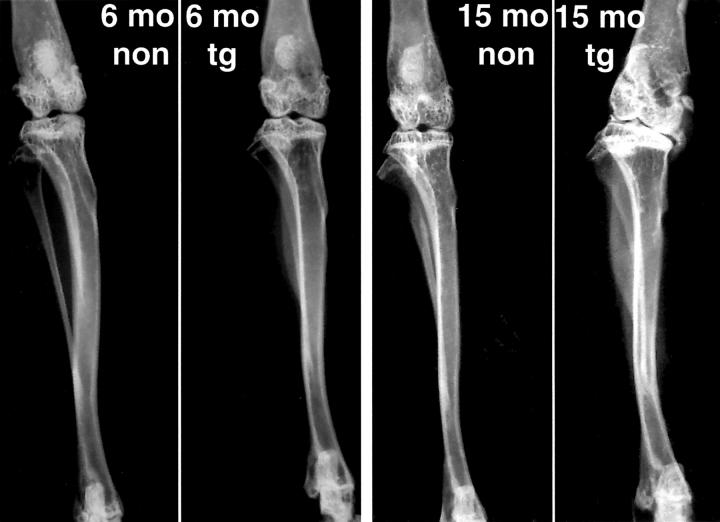
Figure 8 .
Radiographs of the knee joints of non-transgenic (non) and transgenic (tg) Del1 mouse non-runners at the age of 6 and 15 months. Lateral compartments at the left side and medial compartments at the right side. Note the varus deformity and prominent subchondral sclerosis of the knee joint of the 15 month-old transgenic mouse.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arokoski J. P., Hyttinen M. M., Lapveteläinen T., Takács P., Kosztáczky B., Módis L., Kovanen V., Helminen H. Decreased birefringence of the superficial zone collagen network in the canine knee (stifle) articular cartilage after long distance running training, detected by quantitative polarised light microscopy. Ann Rheum Dis. 1996 Apr;55(4):253–264. doi: 10.1136/ard.55.4.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aszódi A., Pfeifer A., Wendel M., Hiripi L., Fässler R. Mouse models for extracellular matrix diseases. J Mol Med (Berl) 1998 Mar;76(3-4):238–252. doi: 10.1007/s001090050214. [DOI] [PubMed] [Google Scholar]
- Cicuttini F. M., Spector T. D. What is the evidence that osteoarthritis is genetically determined? Baillieres Clin Rheumatol. 1997 Nov;11(4):657–669. doi: 10.1016/s0950-3579(97)80002-7. [DOI] [PubMed] [Google Scholar]
- Cooper C., Coggon D. Physical activity and knee osteoarthritis. Lancet. 1999 Jun 26;353(9171):2177–2178. doi: 10.1016/S0140-6736(99)90094-6. [DOI] [PubMed] [Google Scholar]
- Creamer P., Hochberg M. C. Osteoarthritis. Lancet. 1997 Aug 16;350(9076):503–508. doi: 10.1016/S0140-6736(97)07226-7. [DOI] [PubMed] [Google Scholar]
- Dieppe P. Osteoarthritis: time to shift the paradigm. This includes distinguishing between severe disease and common minor disability. BMJ. 1999 May 15;318(7194):1299–1300. doi: 10.1136/bmj.318.7194.1299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fässler R., Schnegelsberg P. N., Dausman J., Shinya T., Muragaki Y., McCarthy M. T., Olsen B. R., Jaenisch R. Mice lacking alpha 1 (IX) collagen develop noninflammatory degenerative joint disease. Proc Natl Acad Sci U S A. 1994 May 24;91(11):5070–5074. doi: 10.1073/pnas.91.11.5070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagg R., Hedbom E., Möllers U., Aszódi A., Fässler R., Bruckner P. Absence of the alpha1(IX) chain leads to a functional knock-out of the entire collagen IX protein in mice. J Biol Chem. 1997 Aug 15;272(33):20650–20654. doi: 10.1074/jbc.272.33.20650. [DOI] [PubMed] [Google Scholar]
- Haimes H. B., Jimenez P. A., Li Y., Shinya T., Olsen B. R. Overexpression of the NC4 domain of type IX collagen induces osteoarthritis in mice. Inflamm Res. 1995 Aug;44 (Suppl 2):S127–S128. doi: 10.1007/BF01778295. [DOI] [PubMed] [Google Scholar]
- Hamerman D. Clinical implications of osteoarthritis and ageing. Ann Rheum Dis. 1995 Feb;54(2):82–85. doi: 10.1136/ard.54.2.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannan M. T., Felson D. T., Anderson J. J., Naimark A. Habitual physical activity is not associated with knee osteoarthritis: the Framingham Study. J Rheumatol. 1993 Apr;20(4):704–709. [PubMed] [Google Scholar]
- Helminen H. J., Kiraly K., Pelttari A., Tammi M. I., Vandenberg P., Pereira R., Dhulipala R., Khillan J. S., Ala-Kokko L., Hume E. L. An inbred line of transgenic mice expressing an internally deleted gene for type II procollagen (COL2A1). Young mice have a variable phenotype of a chondrodysplasia and older mice have osteoarthritic changes in joints. J Clin Invest. 1993 Aug;92(2):582–595. doi: 10.1172/JCI116625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holderbaum D., Haqqi T. M., Moskowitz R. W. Genetics and osteoarthritis: exposing the iceberg. Arthritis Rheum. 1999 Mar;42(3):397–405. doi: 10.1002/1529-0131(199904)42:3<397::AID-ANR1>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- Hyttinen M. M., Töyräs J., Lapveteläinen T., Lindblom J., Prockop D. J., Li S. W., Arita M., Jurvelin J. S., Helminen H. J. Inactivation of one allele of the type II collagen gene alters the collagen network in murine articular cartilage and makes cartilage softer. Ann Rheum Dis. 2001 Mar;60(3):262–268. doi: 10.1136/ard.60.3.262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura T., Nakata K., Tsumaki N., Miyamoto S., Matsui Y., Ebara S., Ochi T. Progressive degeneration of articular cartilage and intervertebral discs. An experimental study in transgenic mice bearing a type IX collagen mutation. Int Orthop. 1996;20(3):177–181. doi: 10.1007/s002640050058. [DOI] [PubMed] [Google Scholar]
- Kirkwood T. B. What is the relationship between osteoarthritis and ageing? Baillieres Clin Rheumatol. 1997 Nov;11(4):683–694. doi: 10.1016/s0950-3579(97)80004-0. [DOI] [PubMed] [Google Scholar]
- Király K., Hyttinen M. M., Lapveteläinen T., Elo M., Kiviranta I., Dobai J., Módis L., Helminen H. J., Arokoski J. P. Specimen preparation and quantification of collagen birefringence in unstained sections of articular cartilage using image analysis and polarizing light microscopy. Histochem J. 1997 Apr;29(4):317–327. doi: 10.1023/a:1020802631968. [DOI] [PubMed] [Google Scholar]
- Kiviranta I., Jurvelin J., Tammi M., Sämänen A. M., Helminen H. J. Microspectrophotometric quantitation of glycosaminoglycans in articular cartilage sections stained with Safranin O. Histochemistry. 1985;82(3):249–255. doi: 10.1007/BF00501401. [DOI] [PubMed] [Google Scholar]
- Kuivaniemi H., Tromp G., Prockop D. J. Mutations in fibrillar collagens (types I, II, III, and XI), fibril-associated collagen (type IX), and network-forming collagen (type X) cause a spectrum of diseases of bone, cartilage, and blood vessels. Hum Mutat. 1997;9(4):300–315. doi: 10.1002/(SICI)1098-1004(1997)9:4<300::AID-HUMU2>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- Kujala U. M., Kettunen J., Paananen H., Aalto T., Battié M. C., Impivaara O., Videman T., Sarna S. Knee osteoarthritis in former runners, soccer players, weight lifters, and shooters. Arthritis Rheum. 1995 Apr;38(4):539–546. doi: 10.1002/art.1780380413. [DOI] [PubMed] [Google Scholar]
- Lane N. E., Hochberg M. C., Pressman A., Scott J. C., Nevitt M. C. Recreational physical activity and the risk of osteoarthritis of the hip in elderly women. J Rheumatol. 1999 Apr;26(4):849–854. [PubMed] [Google Scholar]
- Lapveteläinen T., Hyttinen M., Lindblom J., Långsjö T. K., Sironen R., Li S. W., Arita M., Prockop D. J., Puustjärvi K., Helminen H. J. More knee joint osteoarthritis (OA) in mice after inactivation of one allele of type II procollagen gene but less OA after lifelong voluntary wheel running exercise. Osteoarthritis Cartilage. 2001 Feb;9(2):152–160. doi: 10.1053/joca.2000.0370. [DOI] [PubMed] [Google Scholar]
- Lapveteläinen T., Nevalainen T., Parkkinen J. J., Arokoski J., Kiraly K., Hyttinen M., Halonen P., Helminen H. J. Lifelong moderate running training increases the incidence and severity of osteoarthritis in the knee joint of C57BL mice. Anat Rec. 1995 Jun;242(2):159–165. doi: 10.1002/ar.1092420204. [DOI] [PubMed] [Google Scholar]
- Lapveteläinen T., Tiihonen A., Koskela P., Nevalainen T., Lindblom J., Király K., Halonen P., Helminen H. J. Training a large number of laboratory mice using running wheels and analyzing running behavior by use of a computer-assisted system. Lab Anim Sci. 1997 Apr;47(2):172–179. [PubMed] [Google Scholar]
- Lequesne M. G., Dang N., Lane N. E. Sport practice and osteoarthritis of the limbs. Osteoarthritis Cartilage. 1997 Mar;5(2):75–86. doi: 10.1016/s1063-4584(97)80001-5. [DOI] [PubMed] [Google Scholar]
- Li S. W., Prockop D. J., Helminen H., Fässler R., Lapveteläinen T., Kiraly K., Peltarri A., Arokoski J., Lui H., Arita M. Transgenic mice with targeted inactivation of the Col2 alpha 1 gene for collagen II develop a skeleton with membranous and periosteal bone but no endochondral bone. Genes Dev. 1995 Nov 15;9(22):2821–2830. doi: 10.1101/gad.9.22.2821. [DOI] [PubMed] [Google Scholar]
- Lindberg H., Roos H., Gärdsell P. Prevalence of coxarthrosis in former soccer players. 286 players compared with matched controls. Acta Orthop Scand. 1993 Apr;64(2):165–167. doi: 10.3109/17453679308994561. [DOI] [PubMed] [Google Scholar]
- Martel-Pelletier J. Pathophysiology of osteoarthritis. Osteoarthritis Cartilage. 1998 Nov;6(6):374–376. doi: 10.1053/joca.1998.0140. [DOI] [PubMed] [Google Scholar]
- McAlindon T. E., Wilson P. W., Aliabadi P., Weissman B., Felson D. T. Level of physical activity and the risk of radiographic and symptomatic knee osteoarthritis in the elderly: the Framingham study. Am J Med. 1999 Feb;106(2):151–157. doi: 10.1016/s0002-9343(98)00413-6. [DOI] [PubMed] [Google Scholar]
- Metsäranta M., Garofalo S., Decker G., Rintala M., de Crombrugghe B., Vuorio E. Chondrodysplasia in transgenic mice harboring a 15-amino acid deletion in the triple helical domain of pro alpha 1(II) collagen chain. J Cell Biol. 1992 Jul;118(1):203–212. doi: 10.1083/jcb.118.1.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakata K., Ono K., Miyazaki J., Olsen B. R., Muragaki Y., Adachi E., Yamamura K., Kimura T. Osteoarthritis associated with mild chondrodysplasia in transgenic mice expressing alpha 1(IX) collagen chains with a central deletion. Proc Natl Acad Sci U S A. 1993 Apr 1;90(7):2870–2874. doi: 10.1073/pnas.90.7.2870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen B. R. Collagen IX. Int J Biochem Cell Biol. 1997 Apr;29(4):555–558. doi: 10.1016/s1357-2725(96)00100-8. [DOI] [PubMed] [Google Scholar]
- Prockop D. J., Ala-Kokko L., McLain D. A., Williams C. Can mutated genes cause common osteoarthritis? Br J Rheumatol. 1997 Aug;36(8):827–829. doi: 10.1093/rheumatology/36.8.827. [DOI] [PubMed] [Google Scholar]
- Prockop D. J., Kivirikko K. I. Collagens: molecular biology, diseases, and potentials for therapy. Annu Rev Biochem. 1995;64:403–434. doi: 10.1146/annurev.bi.64.070195.002155. [DOI] [PubMed] [Google Scholar]
- Savontaus M., Metsranta M., Vuorio E. Retarded skeletal development in transgenic mice with a type II collagen mutation. Am J Pathol. 1996 Dec;149(6):2169–2182. [PMC free article] [PubMed] [Google Scholar]
- Spector T. D., Harris P. A., Hart D. J., Cicuttini F. M., Nandra D., Etherington J., Wolman R. L., Doyle D. V. Risk of osteoarthritis associated with long-term weight-bearing sports: a radiologic survey of the hips and knees in female ex-athletes and population controls. Arthritis Rheum. 1996 Jun;39(6):988–995. doi: 10.1002/art.1780390616. [DOI] [PubMed] [Google Scholar]
- Stoop R., van der Kraan P. M., Buma P., Hollander A. P., Poole A. R., van den Berg W. B. Denaturation of type II collagen in articular cartilage in experimental murine arthritis. Evidence for collagen degradation in both reversible and irreversible cartilage damage. J Pathol. 1999 Jul;188(3):329–337. doi: 10.1002/(SICI)1096-9896(199907)188:3<329::AID-PATH371>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- Sämänen A. K., Salminen H. J., Dean P. B., De Crombrugghe B., Vuorio E. I., Metsäranta M. P. Osteoarthritis-like lesions in transgenic mice harboring a small deletion mutation in type II collagen gene. Osteoarthritis Cartilage. 2000 Jul;8(4):248–257. doi: 10.1053/joca.2000.0298. [DOI] [PubMed] [Google Scholar]
- Talts J. F., Pfeifer A., Hofmann F., Hunziker E. B., Zhou X. H., Aszódi A., Fässler R. Endochondral ossification is dependent on the mechanical properties of cartilage tissue and on intracellular signals in chondrocytes. Ann N Y Acad Sci. 1998 Oct 23;857:74–85. doi: 10.1111/j.1749-6632.1998.tb10108.x. [DOI] [PubMed] [Google Scholar]
- Vandenberg P., Vuoristo M. M., Ala-Kokko L., Prockop D. J. The mouse col11a2 gene. Some transcripts from the adjacent rxr-beta gene extend into the col11a2 gene. Matrix Biol. 1996 Nov;15(5):359–367. doi: 10.1016/s0945-053x(96)90139-0. [DOI] [PubMed] [Google Scholar]
- Vikkula M., Olsen B. R. Unravelling the molecular genetics of osteoarthrosis. Ann Med. 1996 Aug;28(4):301–304. doi: 10.3109/07853899608999084. [DOI] [PubMed] [Google Scholar]
- van den Berg W. B. Joint inflammation and cartilage destruction may occur uncoupled. Springer Semin Immunopathol. 1998;20(1-2):149–164. doi: 10.1007/BF00832004. [DOI] [PubMed] [Google Scholar]