Abstract
Objective: To investigate the prognostic value of procollagen type II carboxy-terminal propeptide (PIICP) level in synovial fluid in relation to early tibiofemoral joint osteoarthritis (OA).
Methods: Data were collected on 172 women (age 40 to 59 years) who had knee pain and tibiofemoral joint OA in the early stage. Standing semiflexed knee radiographs were obtained by fluoroscopy at baseline and at four year follow up and a computerised, magnification corrected measurement system was applied to measurement of minimal joint space width in the tibiofemoral compartment. Synovial fluid sampling was performed at baseline and at the four year follow up. Levels of PIICP in the synovial fluid were measured by enzyme immunoassay. The outcome measures were assessed by radiographic joint space narrowing (JSN) in the tibiofemoral joints over four years. Multiple linear regression analyses were used to examine the relation between radiographic JSN and synovial fluid level of PIICP.
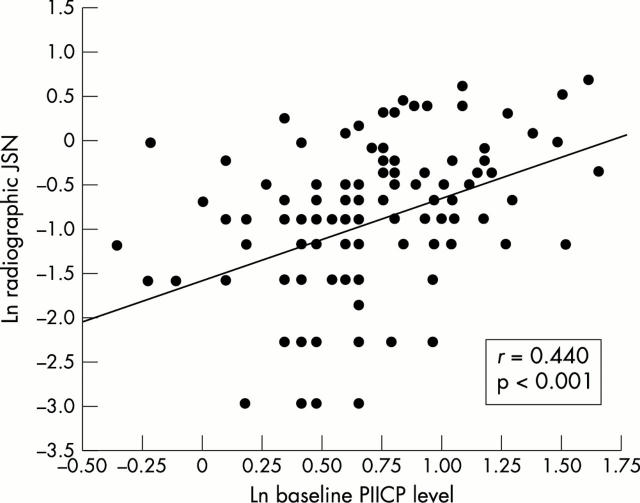
Results: The number of women available at both baseline and at four year follow up was 110. The average of radiographic JSN over four years was 0.53 mm (range 0.00-2.01). Body mass index showed a slightly positive association with baseline PIICP level. In multiple linear regression analyses adjusted for age and body mass index, radiographic JSN over four years had a direct positive correlation with baseline PIICP level (r=0.395; 95% confidence interval (95% CI) 0.231 to 0.529; p<0.001).
Conclusion: In a four year prospective study of women, quantification of synovial fluid PIICP was able to predict subsequent radiographic progression in early tibiofemoral joint OA.
Full Text
The Full Text of this article is available as a PDF (137.6 KB).
Figure 1 .
Correlation between baseline PIICP level (ln transformed data) and radiographic JSN over four years (ln transformed data) in 110 patients with early knee OA.
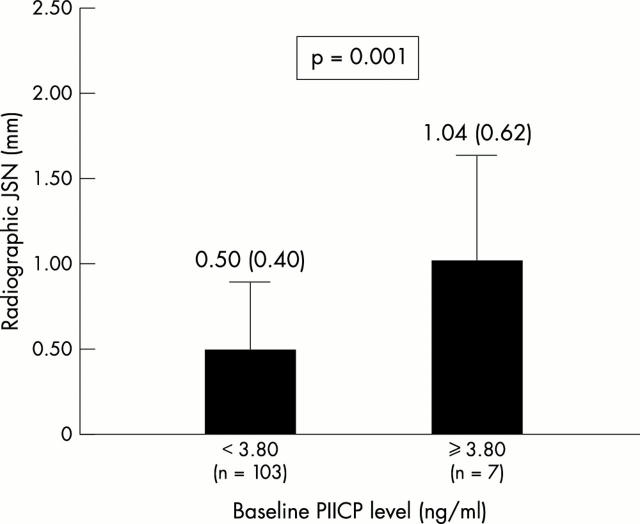
Figure 2 .
Radiographic JSN over four years (mm) according to the baseline PIICP level (ng/ml) (cut off value of 3.80 ng/ml) in 110 patients with early knee OA.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Altman R. D., Hochberg M., Murphy W. A., Jr, Wolfe F., Lequesne M. Atlas of individual radiographic features in osteoarthritis. Osteoarthritis Cartilage. 1995 Sep;3 (Suppl A):3–70. [PubMed] [Google Scholar]
- Buckland-Wright C. Protocols for precise radio-anatomical positioning of the tibiofemoral and patellofemoral compartments of the knee. Osteoarthritis Cartilage. 1995 Sep;3 (Suppl A):71–80. [PubMed] [Google Scholar]
- Buckland-Wright J. C., Macfarlane D. G., Williams S. A., Ward R. J. Accuracy and precision of joint space width measurements in standard and macroradiographs of osteoarthritic knees. Ann Rheum Dis. 1995 Nov;54(11):872–880. doi: 10.1136/ard.54.11.872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckland-Wright Radiographic assessment of osteoarthritis: comparison between existing methodologies. Osteoarthritis Cartilage. 1999 Jul;7(4):430–433. doi: 10.1053/joca.1998.0234. [DOI] [PubMed] [Google Scholar]
- Cooper C., McAlindon T., Coggon D., Egger P., Dieppe P. Occupational activity and osteoarthritis of the knee. Ann Rheum Dis. 1994 Feb;53(2):90–93. doi: 10.1136/ard.53.2.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahlberg L., Billinghurst R. C., Manner P., Nelson F., Webb G., Ionescu M., Reiner A., Tanzer M., Zukor D., Chen J. Selective enhancement of collagenase-mediated cleavage of resident type II collagen in cultured osteoarthritic cartilage and arrest with a synthetic inhibitor that spares collagenase 1 (matrix metalloproteinase 1). Arthritis Rheum. 2000 Mar;43(3):673–682. doi: 10.1002/1529-0131(200003)43:3<673::AID-ANR25>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- Dieppe P. A., Cushnaghan J., Shepstone L. The Bristol 'OA500' study: progression of osteoarthritis (OA) over 3 years and the relationship between clinical and radiographic changes at the knee joint. Osteoarthritis Cartilage. 1997 Mar;5(2):87–97. doi: 10.1016/s1063-4584(97)80002-7. [DOI] [PubMed] [Google Scholar]
- Dougados M., Gueguen A., Nguyen M., Thiesce A., Listrat V., Jacob L., Nakache J. P., Gabriel K. R., Lequesne M., Amor B. Longitudinal radiologic evaluation of osteoarthritis of the knee. J Rheumatol. 1992 Mar;19(3):378–384. [PubMed] [Google Scholar]
- Fawthrop F., Yaqub R., Belcher C., Bayliss M., Ledingham J., Doherty M. Chondroitin and keratan sulphate epitopes, glycosaminoglycans, and hyaluronan in progressive versus non-progressive osteoarthritis. Ann Rheum Dis. 1997 Feb;56(2):119–122. doi: 10.1136/ard.56.2.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fife R. S., Brandt K. D., Braunstein E. M., Katz B. P., Shelbourne K. D., Kalasinski L. A., Ryan S. Relationship between arthroscopic evidence of cartilage damage and radiographic evidence of joint space narrowing in early osteoarthritis of the knee. Arthritis Rheum. 1991 Apr;34(4):377–382. doi: 10.1002/art.1780340402. [DOI] [PubMed] [Google Scholar]
- Hollander A. P., Heathfield T. F., Webber C., Iwata Y., Bourne R., Rorabeck C., Poole A. R. Increased damage to type II collagen in osteoarthritic articular cartilage detected by a new immunoassay. J Clin Invest. 1994 Apr;93(4):1722–1732. doi: 10.1172/JCI117156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollander A. P., Pidoux I., Reiner A., Rorabeck C., Bourne R., Poole A. R. Damage to type II collagen in aging and osteoarthritis starts at the articular surface, originates around chondrocytes, and extends into the cartilage with progressive degeneration. J Clin Invest. 1995 Dec;96(6):2859–2869. doi: 10.1172/JCI118357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi T., Yoshihara Y., Samura A., Yamada H., Shinmei M., Roos H., Lohmander L. S. Synovial fluid concentrations of the C-propeptide of type II collagen correlate with body mass index in primary knee osteoarthritis. Ann Rheum Dis. 1997 Aug;56(8):500–503. doi: 10.1136/ard.56.8.500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lohmander L. S., Yoshihara Y., Roos H., Kobayashi T., Yamada H., Shinmei M. Procollagen II C-propeptide in joint fluid: changes in concentration with age, time after knee injury, and osteoarthritis. J Rheumatol. 1996 Oct;23(10):1765–1769. [PubMed] [Google Scholar]
- Mazzuca S. A., Brandt K. D., Katz B. P. Is conventional radiography suitable for evaluation of a disease-modifying drug in patients with knee osteoarthritis? Osteoarthritis Cartilage. 1997 Jul;5(4):217–226. doi: 10.1016/s1063-4584(97)80017-9. [DOI] [PubMed] [Google Scholar]
- Nelson F., Dahlberg L., Laverty S., Reiner A., Pidoux I., Ionescu M., Fraser G. L., Brooks E., Tanzer M., Rosenberg L. C. Evidence for altered synthesis of type II collagen in patients with osteoarthritis. J Clin Invest. 1998 Dec 15;102(12):2115–2125. doi: 10.1172/JCI4853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niyibizi C., Wu J. J., Eyre D. R. The carboxypropeptide trimer of type II collagen is a prominent component of immature cartilages and intervertebral-disc tissue. Biochim Biophys Acta. 1987 Dec 18;916(3):493–499. doi: 10.1016/0167-4838(87)90196-8. [DOI] [PubMed] [Google Scholar]
- Oliveria S. A., Felson D. T., Reed J. I., Cirillo P. A., Walker A. M. Incidence of symptomatic hand, hip, and knee osteoarthritis among patients in a health maintenance organization. Arthritis Rheum. 1995 Aug;38(8):1134–1141. doi: 10.1002/art.1780380817. [DOI] [PubMed] [Google Scholar]
- Peltonen L., Halila R., Ryhänen L. Enzymes converting procollagens to collagens. J Cell Biochem. 1985;28(1):15–21. doi: 10.1002/jcb.240280104. [DOI] [PubMed] [Google Scholar]
- Schouten J. S., van den Ouweland F. A., Valkenburg H. A. A 12 year follow up study in the general population on prognostic factors of cartilage loss in osteoarthritis of the knee. Ann Rheum Dis. 1992 Aug;51(8):932–937. doi: 10.1136/ard.51.8.932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharif M., George E., Shepstone L., Knudson W., Thonar E. J., Cushnaghan J., Dieppe P. Serum hyaluronic acid level as a predictor of disease progression in osteoarthritis of the knee. Arthritis Rheum. 1995 Jun;38(6):760–767. doi: 10.1002/art.1780380608. [DOI] [PubMed] [Google Scholar]
- Sharif M., Saxne T., Shepstone L., Kirwan J. R., Elson C. J., Heinegård D., Dieppe P. A. Relationship between serum cartilage oligomeric matrix protein levels and disease progression in osteoarthritis of the knee joint. Br J Rheumatol. 1995 Apr;34(4):306–310. doi: 10.1093/rheumatology/34.4.306. [DOI] [PubMed] [Google Scholar]
- Shinmei M., Ito K., Matsuyama S., Yoshihara Y., Matsuzawa K. Joint fluid carboxy-terminal type II procollagen peptide as a marker of cartilage collagen biosynthesis. Osteoarthritis Cartilage. 1993 Apr;1(2):121–128. doi: 10.1016/s1063-4584(05)80027-5. [DOI] [PubMed] [Google Scholar]
- Shinmei M., Kobayashi T., Yoshihara Y., Samura A. Significance of the levels of carboxy terminal type II procollagen peptide, chondroitin sulfate isomers, tissue inhibitor of metalloproteinases, and metalloproteinases in osteoarthritis joint fluid. J Rheumatol Suppl. 1995 Feb;43:78–81. [PubMed] [Google Scholar]
- Spector T. D., Hart D. J., Nandra D., Doyle D. V., Mackillop N., Gallimore J. R., Pepys M. B. Low-level increases in serum C-reactive protein are present in early osteoarthritis of the knee and predict progressive disease. Arthritis Rheum. 1997 Apr;40(4):723–727. doi: 10.1002/art.1780400419. [DOI] [PubMed] [Google Scholar]
- Stoop R., van der Kraan P. M., Buma P., Hollander A. P., Billinghurst R. C., Poole A. R., van den Berg W. B. Type II collagen degradation in spontaneous osteoarthritis in C57Bl/6 and BALB/c mice. Arthritis Rheum. 1999 Nov;42(11):2381–2389. doi: 10.1002/1529-0131(199911)42:11<2381::AID-ANR17>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]