Abstract
Background: Raised interleukin (IL)6 and IL10 levels are thought to contribute to the pathogenesis of systemic lupus erythematosus (SLE) by enhancing autoantibody production and immune complex (IC) formation. These immune complexes can then stimulate cellular reactions through Fc and complement receptors.
Objective: To investigate whether circulating SLE ICs stimulate type 2 cytokine production.
Methods: Twenty serum samples from patients with active SLE were compared with sera from 18 healthy controls. Sera and polyethylene glycol (PEG) precipitates from sera were added to peripheral blood mononuclear cell (PBMC) cultures, and the production of IL10 and IL6 was investigated by enzyme linked immunospot assay (ELISPOT) and enzyme linked immunosorbent assay (ELISA). Fc gamma receptor (FcγR) antibodies were used in blocking experiments, and flow cytometry was used to assess the correlation between monocyte FcγR expression and IC-induced cytokine production.
Results: Ten per cent dilutions of the SLE sera induced a significantly increased number of IL10-producing cells in comparison with control sera (median, 11.75 v 1.25 spot forming cells/50 000 PBMC; p<0.0001). PEG precipitates from SLE sera also induced significantly increased levels of IL10 (p=0.016) and IL6 (p=0.042) in comparison with control PEG precipitates. IL10 production induced by SLE PEG precipitates or by artificial ICs could be blocked by anti-FcγRII antibodies, and the FcγRII expression on CD14+ monocytes correlated with the IC-induced production of IL10 and IL6.
Conclusions: SLE sera stimulate IL10 and IL6 production from PBMC, and this effect is at least partly explained by precipitable ICs acting through FcγRII. This effect provides a possible mechanism for the enhanced production of IL10 in SLE, whereby B cell activation, antibody production, IC stimulated monocytes/macrophages, and type 2 cytokines create a vicious cycle that may help to maintain B cell hyperactivity in SLE.
Full Text
The Full Text of this article is available as a PDF (213.4 KB).
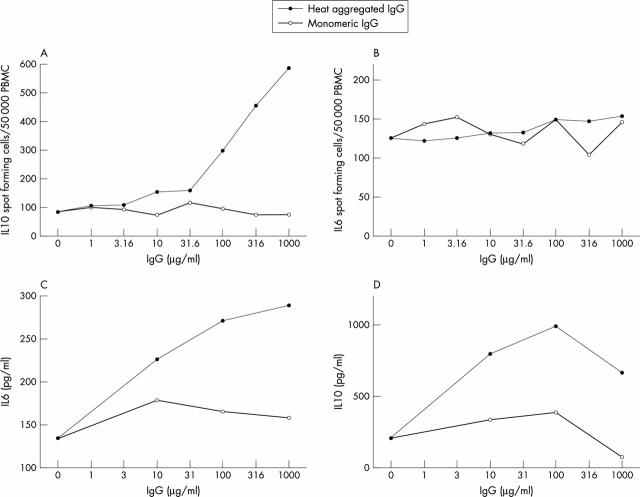
Figure 1 .
Heat aggregated IgG induces production of IL10 and IL6 in cultures of human PBMC by increasing the number of cells producing IL10, but not IL6. Human monomeric IgG was heat aggregated and diluted to various concentrations in PBS, and then added to PBMC cultures (5x105, 2x104, and 1x106 PBMC/ml for IL10 ELISPOT, IL6 ELISPOT, and supernatants, respectively) in complete medium and incubated overnight. As a control, non-aggregated IgG was used. (A) and (B) show the number of IL10 and IL6 spot forming cells, respectively. In parallel cultures, supernatant levels of (C) IL10 and (D) IL6 were determined by ELISA. These results are representative of five experiments for ELISPOT and supernatants respectively, using PBMC from different healthy donors.
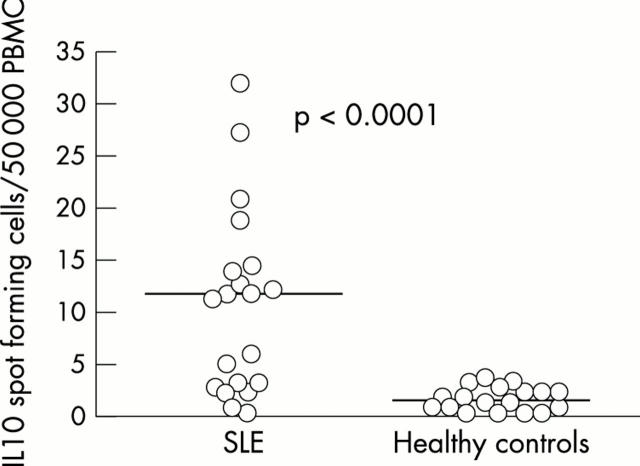
Figure 2 .
SLE sera stimulate an increase in the number of IL10 SFC. Twenty serum samples from patients with SLE and 18 sera from healthy controls (10% of final volume) were added to 100 µl PBMC suspensions from a healthy donor (5x105 cells/ml) in duplicate. After 20 hours of incubation, the number of IL10 producing cells was enumerated by the ELISPOT technique. The results are representative of two experiment using the same sera and cell donor, with comparable results. Horizontal bars show the median values for each group.
Figure 3 .
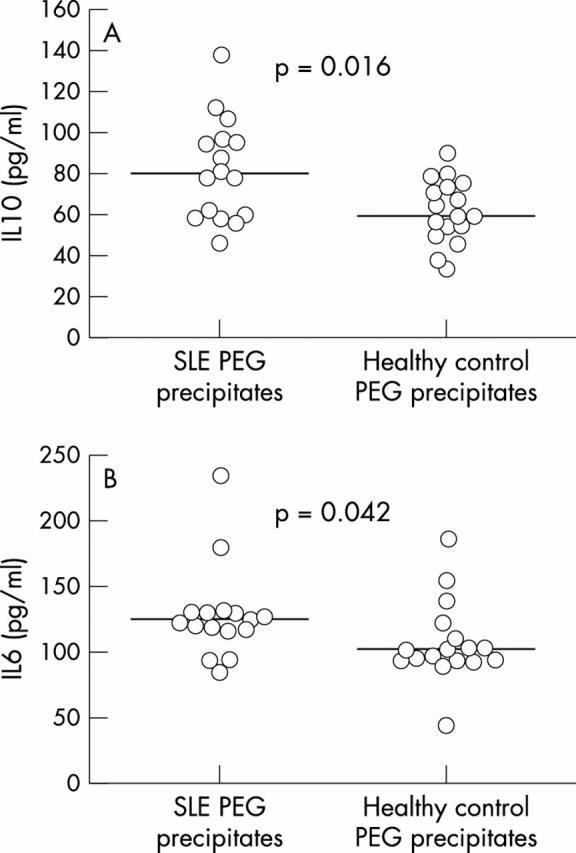
High molecular weight PEG precipitates from SLE sera induce IL10 and IL6 production in human PBMC cultures. Serum samples from 16 patients with SLE and 17 healthy controls were subjected to PEG precipitation, and the precipitates were diluted to the original serum volume in PBS. Ten per cent (final concentration) of each precipitate was added to a 250 µl suspension of PBMC (1x106 cells/ml) from the healthy subject investigated in fig 2. After incubation for 20 hours, supernatant levels of (A) IL10 and (B) IL6 were assessed by ELISA. Horizontal bars show the median values for each group. Owing to a shortage of serum, not all of the samples studied in fig 2 were subject to PEG precipitation.
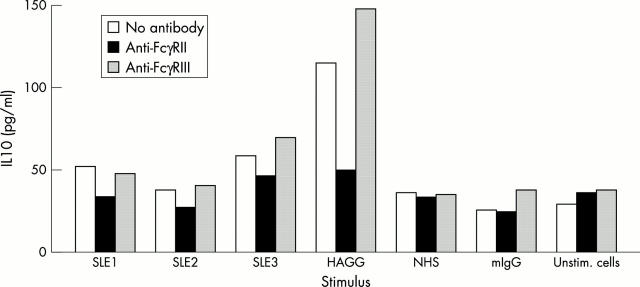
Figure 4 .
Effects of blockade of FcγRII and FcγRIII on IC-induced cytokine production. Cell cultures were incubated with 1.5 µg/ml of blocking Fab (FcγRII) or F(ab‘)2 (FcγRIII) fragments for 30 minutes before addition of stimulus. PBMC (106/ml) from the healthy donor used in figs 2 and 3 were stimulated with PEG precipitates from three active SLE sera or with HAGG (100 µg/ml), or with a PEG precipitate from a healthy control (NHS), with monomeric IgG (mIgG; 100 µg/ml), or with unstimulated cells. Supernatants were collected after 20 hours' incubation and levels of IL10 analysed by ELISA.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Agnello V., Winchester R. J., Kunkel H. G. Precipitin reactions of the C1q component of complement with aggregated gamma-globulin and immune complexes in gel diffusion. Immunology. 1970 Dec;19(6):909–919. [PMC free article] [PubMed] [Google Scholar]
- Batteux F., Palmer P., Daëron M., Weill B., Lebon P. FCgammaRII (CD32)-dependent induction of interferon-alpha by serum from patients with lupus erythematosus. Eur Cytokine Netw. 1999 Dec;10(4):509–514. [PubMed] [Google Scholar]
- Berg L., Rönnelid J., Sanjeevi C. B., Lampa J., Klareskog L. Interferon-gamma production in response to in vitro stimulation with collagen type II in rheumatoid arthritis is associated with HLA-DRB1(*)0401 and HLA-DQ8. Arthritis Res. 2000;2(1):75–84. doi: 10.1186/ar71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger S., Balló H., Stutte H. J. Distinct antigen-induced cytokine pattern upon stimulation with antibody-complexed antigen consistent with a Th1-->Th2-shift. Res Virol. 1996 Mar-Jun;147(2-3):103–108. doi: 10.1016/0923-2516(96)80223-8. [DOI] [PubMed] [Google Scholar]
- Berger S., Balló H., Stutte H. J. Immune complex-induced interleukin-6, interleukin-10 and prostaglandin secretion by human monocytes: a network of pro- and anti-inflammatory cytokines dependent on the antigen:antibody ratio. Eur J Immunol. 1996 Jun;26(6):1297–1301. doi: 10.1002/eji.1830260618. [DOI] [PubMed] [Google Scholar]
- Berger S., Chandra R., Balló H., Hildenbrand R., Stutte H. J. Immune complexes are potent inhibitors of interleukin-12 secretion by human monocytes. Eur J Immunol. 1997 Nov;27(11):2994–3000. doi: 10.1002/eji.1830271136. [DOI] [PubMed] [Google Scholar]
- Blanco P., Palucka A. K., Gill M., Pascual V., Banchereau J. Induction of dendritic cell differentiation by IFN-alpha in systemic lupus erythematosus. Science. 2001 Nov 16;294(5546):1540–1543. doi: 10.1126/science.1064890. [DOI] [PubMed] [Google Scholar]
- Feng Xuan, Yau Douglas, Holbrook Christopher, Reder Anthony T. Type I interferons inhibit interleukin-10 production in activated human monocytes and stimulate IL-10 in T cells: implications for Th1-mediated diseases. J Interferon Cytokine Res. 2002 Mar;22(3):311–319. doi: 10.1089/107999002753675730. [DOI] [PubMed] [Google Scholar]
- Fuchs A. C., Granowitz E. V., Shapiro L., Vannier E., Lonnemann G., Angel J. B., Kennedy J. S., Rabson A. R., Radwanski E., Affrime M. B. Clinical, hematologic, and immunologic effects of interleukin-10 in humans. J Clin Immunol. 1996 Sep;16(5):291–303. doi: 10.1007/BF01541395. [DOI] [PubMed] [Google Scholar]
- Gröndal G., Gunnarsson I., Rönnelid J., Rogberg S., Klareskog L., Lundberg I. Cytokine production, serum levels and disease activity in systemic lupus erythematosus. Clin Exp Rheumatol. 2000 Sep-Oct;18(5):565–570. [PubMed] [Google Scholar]
- Gröndal G., Kristjansdottir H., Gunnlaugsdottir B., Arnason A., Lundberg I., Klareskog L., Steinsson K. Increased number of interleukin-10-producing cells in systemic lupus erythematosus patients and their first-degree relatives and spouses in Icelandic multicase families. Arthritis Rheum. 1999 Aug;42(8):1649–1654. doi: 10.1002/1529-0131(199908)42:8<1649::AID-ANR13>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- Hagiwara E., Gourley M. F., Lee S., Klinman D. K. Disease severity in patients with systemic lupus erythematosus correlates with an increased ratio of interleukin-10:interferon-gamma-secreting cells in the peripheral blood. Arthritis Rheum. 1996 Mar;39(3):379–385. doi: 10.1002/art.1780390305. [DOI] [PubMed] [Google Scholar]
- Houssiau F. A., Lefebvre C., Vanden Berghe M., Lambert M., Devogelaer J. P., Renauld J. C. Serum interleukin 10 titers in systemic lupus erythematosus reflect disease activity. Lupus. 1995 Oct;4(5):393–395. doi: 10.1177/096120339500400510. [DOI] [PubMed] [Google Scholar]
- Lacki J. K., Klama K., Porawska W., Mackiewicz S. H., Müller W., Wiktorowicz K. Interleukin 10 inhibits interleukin 6 production and acute phase response in rheumatoid arthritis. Arch Immunol Ther Exp (Warsz) 1995;43(1):11–14. [PubMed] [Google Scholar]
- Lacki J. K., Samborski W., Mackiewicz S. H. Interleukin-10 and interleukin-6 in lupus erythematosus and rheumatoid arthritis, correlations with acute phase proteins. Clin Rheumatol. 1997 May;16(3):275–278. doi: 10.1007/BF02238963. [DOI] [PubMed] [Google Scholar]
- Linker-Israeli M., Honda M., Nand R., Mandyam R., Mengesha E., Wallace D. J., Metzger A., Beharier B., Klinenberg J. R. Exogenous IL-10 and IL-4 down-regulate IL-6 production by SLE-derived PBMC. Clin Immunol. 1999 Apr;91(1):6–16. doi: 10.1006/clim.1998.4680. [DOI] [PubMed] [Google Scholar]
- Llorente L., Richaud-Patin Y., Fior R., Alcocer-Varela J., Wijdenes J., Fourrier B. M., Galanaud P., Emilie D. In vivo production of interleukin-10 by non-T cells in rheumatoid arthritis, Sjögren's syndrome, and systemic lupus erythematosus. A potential mechanism of B lymphocyte hyperactivity and autoimmunity. Arthritis Rheum. 1994 Nov;37(11):1647–1655. doi: 10.1002/art.1780371114. [DOI] [PubMed] [Google Scholar]
- Llorente L., Richaud-Patin Y., Wijdenes J., Alcocer-Varela J., Maillot M. C., Durand-Gasselin I., Fourrier B. M., Galanaud P., Emilie D. Spontaneous production of interleukin-10 by B lymphocytes and monocytes in systemic lupus erythematosus. Eur Cytokine Netw. 1993 Nov-Dec;4(6):421–427. [PubMed] [Google Scholar]
- Llorente L., Zou W., Levy Y., Richaud-Patin Y., Wijdenes J., Alcocer-Varela J., Morel-Fourrier B., Brouet J. C., Alarcon-Segovia D., Galanaud P. Role of interleukin 10 in the B lymphocyte hyperactivity and autoantibody production of human systemic lupus erythematosus. J Exp Med. 1995 Mar 1;181(3):839–844. doi: 10.1084/jem.181.3.839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manger K., Repp R., Spriewald B. M., Rascu A., Geiger A., Wassmuth R., Westerdaal N. A., Wentz B., Manger B., Kalden J. R. Fcgamma receptor IIa polymorphism in Caucasian patients with systemic lupus erythematosus: association with clinical symptoms. Arthritis Rheum. 1998 Jul;41(7):1181–1189. doi: 10.1002/1529-0131(199807)41:7<1181::AID-ART6>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- Mongan A. E., Ramdahin S., Warrington R. J. Interleukin-10 response abnormalities in systemic lupus erythematosus. Scand J Immunol. 1997 Oct;46(4):406–412. doi: 10.1046/j.1365-3083.1997.d01-140.x. [DOI] [PubMed] [Google Scholar]
- Nilsson U. R., Nilsson B. Simplified assays of hemolytic activity of the classical and alternative complement pathways. J Immunol Methods. 1984 Aug 3;72(1):49–59. doi: 10.1016/0022-1759(84)90432-0. [DOI] [PubMed] [Google Scholar]
- Norsworthy P., Theodoridis E., Botto M., Athanassiou P., Beynon H., Gordon C., Isenberg D., Walport M. J., Davies K. A. Overrepresentation of the Fcgamma receptor type IIA R131/R131 genotype in caucasoid systemic lupus erythematosus patients with autoantibodies to C1q and glomerulonephritis. Arthritis Rheum. 1999 Sep;42(9):1828–1832. doi: 10.1002/1529-0131(199909)42:9<1828::AID-ANR6>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- Park Y. B., Lee S. K., Kim D. S., Lee J., Lee C. H., Song C. H. Elevated interleukin-10 levels correlated with disease activity in systemic lupus erythematosus. Clin Exp Rheumatol. 1998 May-Jun;16(3):283–288. [PubMed] [Google Scholar]
- Robak E., Sysa-Jedrzejowska A., Stepień H., Robak T. Circulating interleukin-6 type cytokines in patients with systemic lupus erythematosus. Eur Cytokine Netw. 1997 Sep;8(3):281–286. [PubMed] [Google Scholar]
- Rönnblom L., Alm G. V. An etiopathogenic role for the type I IFN system in SLE. Trends Immunol. 2001 Aug;22(8):427–431. doi: 10.1016/s1471-4906(01)01955-x. [DOI] [PubMed] [Google Scholar]
- Rönnelid J., Huang Y. H., Norrlander T., Rogberg S., Nilsson B., Gustafsson R., Klareskog L. Short-term kinetics of the humoral anti-C1q response in SLE using the ELISPOT method: fast decline in production in response to steroids. Scand J Immunol. 1994 Aug;40(2):243–250. doi: 10.1111/j.1365-3083.1994.tb03457.x. [DOI] [PubMed] [Google Scholar]
- Rönnelid J., Klareskog L. A comparison between ELISPOT methods for the detection of cytokine producing cells: greater sensitivity and specificity using ELISA plates as compared to nitrocellulose membranes. J Immunol Methods. 1997 Jan 15;200(1-2):17–26. doi: 10.1016/s0022-1759(96)00170-6. [DOI] [PubMed] [Google Scholar]
- Shanley T. P., Schmal H., Friedl H. P., Jones M. L., Ward P. A. Regulatory effects of intrinsic IL-10 in IgG immune complex-induced lung injury. J Immunol. 1995 Apr 1;154(7):3454–3460. [PubMed] [Google Scholar]
- Tan E. M., Cohen A. S., Fries J. F., Masi A. T., McShane D. J., Rothfield N. F., Schaller J. G., Talal N., Winchester R. J. The 1982 revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum. 1982 Nov;25(11):1271–1277. doi: 10.1002/art.1780251101. [DOI] [PubMed] [Google Scholar]
- Tanguay S., Killion J. J. Direct comparison of ELISPOT and ELISA-based assays for detection of individual cytokine-secreting cells. Lymphokine Cytokine Res. 1994 Aug;13(4):259–263. [PubMed] [Google Scholar]
- Tripp C. S., Beckerman K. P., Unanue E. R. Immune complexes inhibit antimicrobial responses through interleukin-10 production. Effects in severe combined immunodeficient mice during Listeria infection. J Clin Invest. 1995 Apr;95(4):1628–1634. doi: 10.1172/JCI117837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viallard J. F., Pellegrin J. L., Ranchin V., Schaeverbeke T., Dehais J., Longy-Boursier M., Ragnaud J. M., Leng B., Moreau J. F. Th1 (IL-2, interferon-gamma (IFN-gamma)) and Th2 (IL-10, IL-4) cytokine production by peripheral blood mononuclear cells (PBMC) from patients with systemic lupus erythematosus (SLE). Clin Exp Immunol. 1999 Jan;115(1):189–195. doi: 10.1046/j.1365-2249.1999.00766.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wanidworanun C., Strober W. Predominant role of tumor necrosis factor-alpha in human monocyte IL-10 synthesis. J Immunol. 1993 Dec 15;151(12):6853–6861. [PubMed] [Google Scholar]
- al-Janadi M., al-Dalaan A., al-Balla S., al-Humaidi M., Raziuddin S. Interleukin-10 (IL-10) secretion in systemic lupus erythematosus and rheumatoid arthritis: IL-10-dependent CD4+CD45RO+ T cell-B cell antibody synthesis. J Clin Immunol. 1996 Jul;16(4):198–207. doi: 10.1007/BF01541225. [DOI] [PubMed] [Google Scholar]
- van der Pol W., van de Winkel J. G. IgG receptor polymorphisms: risk factors for disease. Immunogenetics. 1998 Aug;48(3):222–232. doi: 10.1007/s002510050426. [DOI] [PubMed] [Google Scholar]