Abstract
Background: Transgenic deficiency in interferon γ (IFNγ) or IFNγ receptor makes resistant strains of mice bearing H-2b or H-2d susceptible to collagen induced arthritis (CIA).
Objective: To determine whether the escape from regulation of disease susceptibility at the major histocompatibility complex level involves a new use of autoimmune T cells expressing T cell receptor (TCR) Vß that vary from the cell populations previously identified within arthritic joints.
Methods: Arthritis was induced by a standard protocol with type II bovine collagen (CII) in complete Freund's adjuvant. Clinical features, histopathology, immunological responses, and TCR profile in arthritic joints in IFNγ knockout C57BL/6 (B6.IFNγ KO) mice (H-2b) were compared directly with those in DBA/1 mice (H-2q).
Results: 60–80% of B6.IFNγ KO mice developed a progressive arthritis with a similar clinical course to classical CIA in DBA/1 mice. The affected joints in B6.IFNγ KO mice had an erosive form of arthritis with similar features to joint disease in DBA/1 mice. B6.IFNγ KO mice produced significantly higher levels of IgG2b and IgG1 autoantibodies to murine CII and showed increased proliferative response to CII compared with B6 mice. Comparable levels of interleukin 1ß and tumour necrosis factor α expression were detected in arthritic joints from ß6.IFNγ KO and DBA/1 mice. B6.IFNγKO mice used predominantly TCR Vß6 and Vß8 in arthritic joints. This TCR Vß profile is similar to that found in DBA/1 mice with CIA.
Conclusions: C57BL/6 mice deficient in IFNγ production can develop arthritis that resembles classical CIA. These data suggest that IFNγ is a key factor mediating susceptibility to CIA.
Full Text
The Full Text of this article is available as a PDF (262.5 KB).
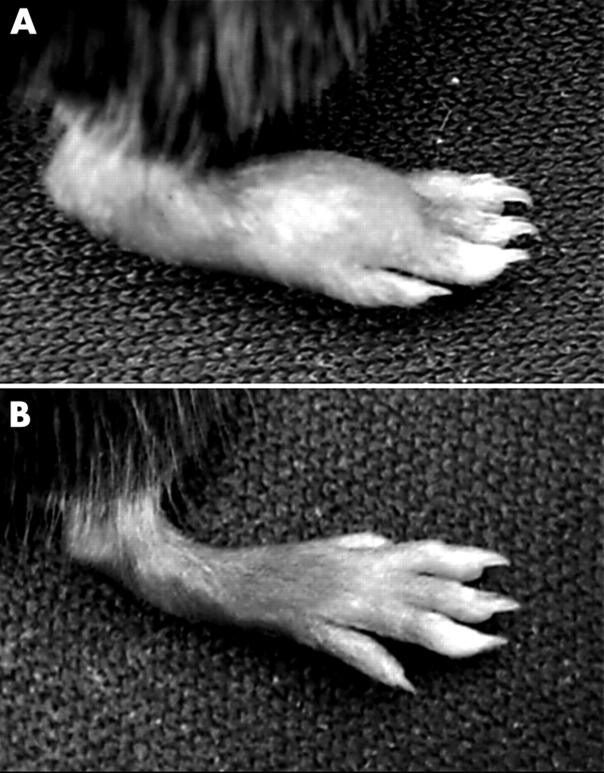
Figure 1.
Arthritis in B6.IFNγ KO mice immunised with bovine CII in CFA. (A) An arthritic hind paw of a B6.IFNγ KO mouse at day 3 of arthritis with a clinical score 1. There is substantial erythematous oedema from the ankle through the digits. Note the marked swollen tarsal and metatarsal joints. This is the typical sign of an arthritic paw seen in CIA in DBA/1 mice. (B) Normal appearance of a non-arthritic hind paw of a B6 mouse immunised with bovine CII in CFA.
Figure 2.
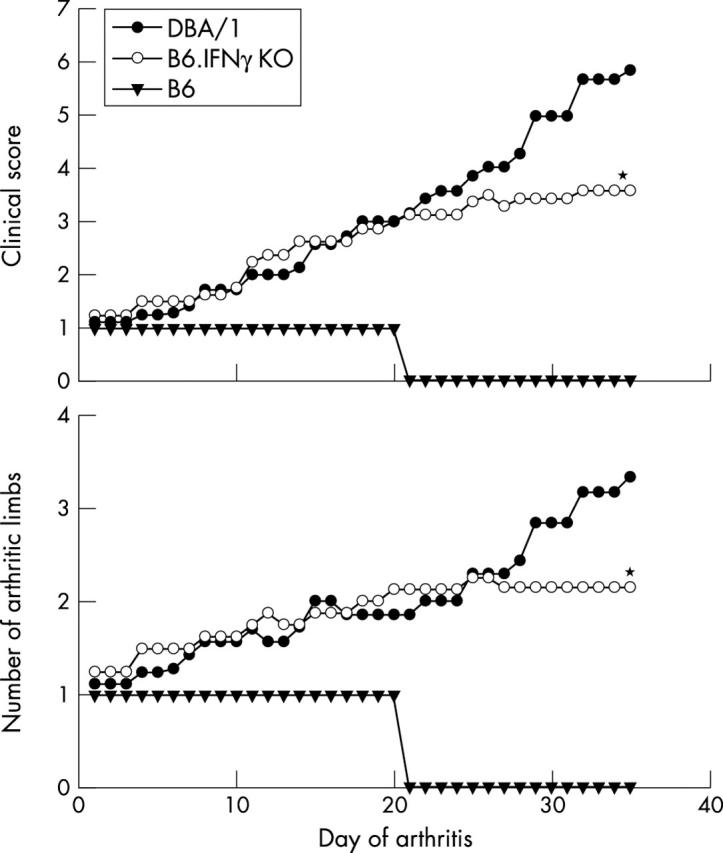
Natural clinical course of arthritis in B6.IFNγ KO mice. The average clinical score of arthritic mice reflects the clinical severity of an individual arthritic limb and the number of affected limbs. DBA/1 mice exhibited a typical monophasic progressive disease. Similarly, B6.IFNγ KO mice showed a progressive type of arthritis. Most of the affected joints progressed from initial swelling to distortion and some affected joints progressed to ankylosis. The lower clinical score at the late stage of disease in B6.IFNγ KO mice reflects the smaller number of limbs affected and less severity of the affected limbs than in DBA/1 mice (*p<0.05). B6 mice had a mild and transient arthritis affecting only one limb in each arthritic mouse.
Figure 3.
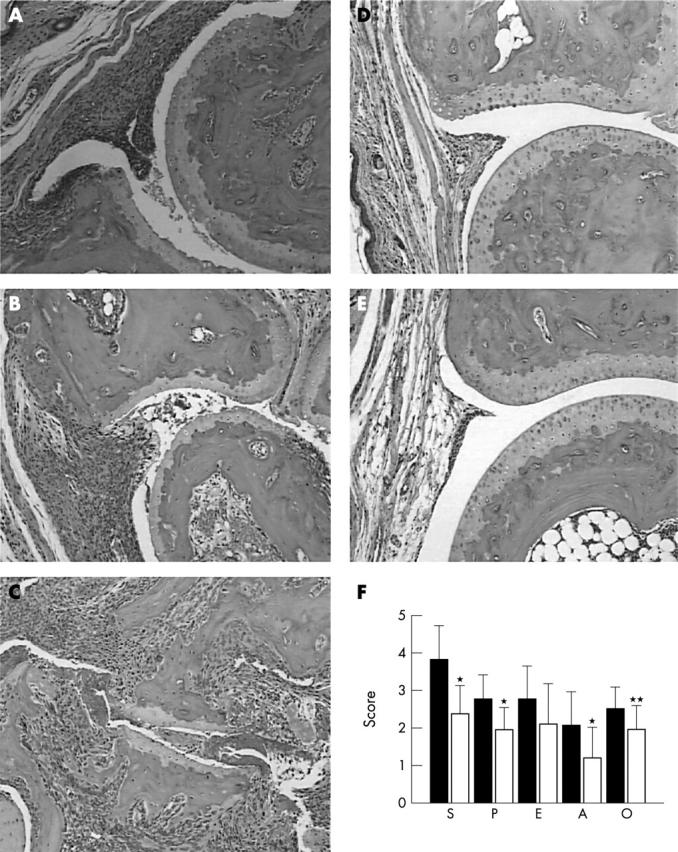
Histopathology of arthritis in B6.IFNγ KO mice. (A) Section of a hind paw with arthritis at the day of onset showing inflamed synovium with massive infiltrate of mononuclear cells. There is decreased number of chondrocytes in the articular cartilage. (B) Section of a hind paw with arthritis at day 14 showing severe synovitis, pannus formation, and erosion and some visible destruction of articular cartilage. (C) Section of a hind paw with arthritis at day 35 showing massive synovitis, erosion of cartilage and subchondral bone, and destruction of joint structure. (D) Normal appearance of a section of a hind paw from a non-arthritic CII immunised B6 mouse. (E) Section of a remitted arthritic hind paw from a B6 mouse showing normal joint appearance. (F) Histological assessment of arthritis in affected limbs in B6.IFNγ KO mice (open bars) at the end of the experiments (day 35 of arthritis) in comparison with that in DBA/1 mice (filled bars). S, synovitis; P, pannus formation; E, erosion; A, architectural changes; O, overall histological score. *p<0.01; **p<0.05.
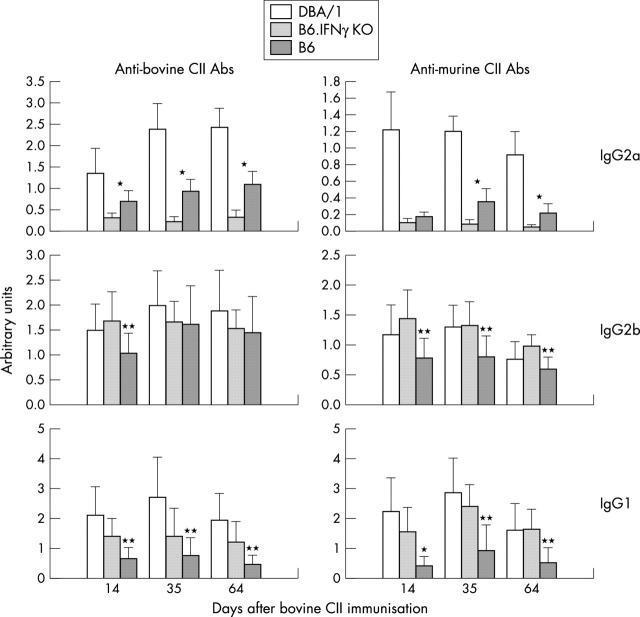
Figure 4.
Levels of anti-heterologous and autologous CII antibodies in B6.IFNγ KO mice immunised with bovine CII in CFA (n=10 mice in each group). *p<0.01 (B6.IFNγ KO v B6); **p<0.05 (B6.IFNγ KO v B6).
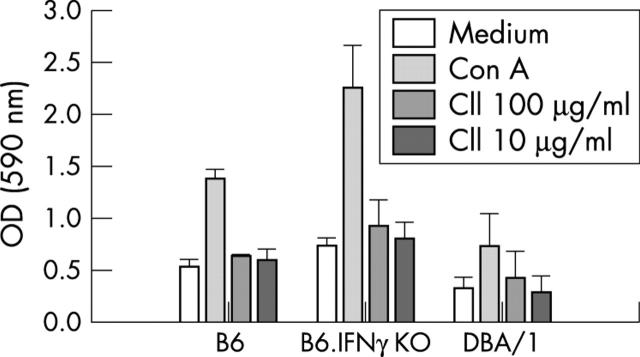
Figure 5.
Enhanced proliferation of lymphocytes from CII immunised B6.IFNγ KO mice. Results are representative of three experiments; n=3 in each group.
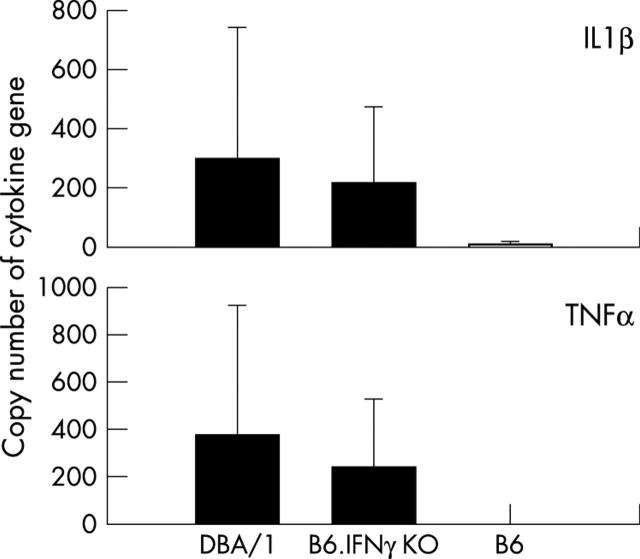
Figure 6.
Expression of cytokines in arthritic joints in B6.IFNγ KO mice. IL1ß and TNFα gene expression was quantified using a quantitative real-time RT-PCR. Levels of IL1ß and TNFα gene expression in arthritic joints in B6.IFNγ KO are comparable with those in DBA/1 mice (p>0.05). Affected and remitted joints in B6 mice had negligible levels of expression of IL1ß and TNFα (p<0.01) compared with those in DBA/1 or B6.IFNγ KO mice.
Figure 7.
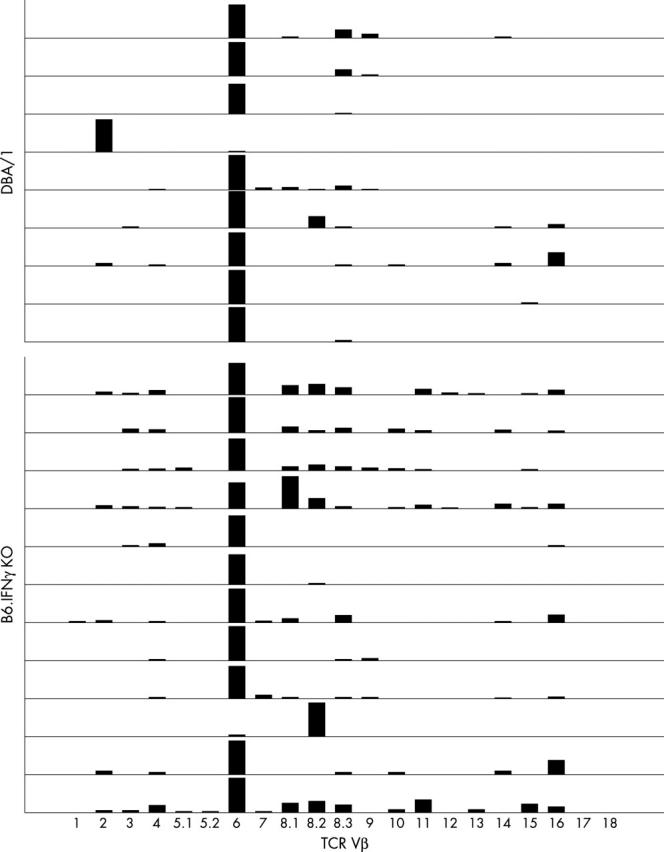
Predominant TCR Vß6 and Vß8 usage in arthritic joints in B6.IFNγ KO mice. Expression of TCR Vß gene expression was determined by quantitative real-time RT-PCR. This composite graph shows individual arthritic mouse samples from both DBA/1 (top, n=9) and B6.IFNγ KO (bottom, n=12) mice. The Vß gene expression was expressed as a relative quantity in reference to the Cß expression in the same sample. In DBA/1 mice, Vß6 had a predominant expression over the other Vß genes in most of the samples. In B6.IFNγ KO mice, Vß6 had a two- to 50-fold increased expression over other Vß genes in 10 of 12 samples. In addition, the Vß8 gene family exhibited the second most increased expression in most Vß6 predominant samples. In the two samples in which Vß6 was not predominant, Vß8.1 and Vß8.2 outnumbered Vß6 as the predominantly expressed Vß genes.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Banerjee S., Haqqi T. M., Luthra H. S., Stuart J. M., David C. S. Possible role of V beta T cell receptor genes in susceptibility to collagen-induced arthritis in mice. J Exp Med. 1988 Mar 1;167(3):832–839. doi: 10.1084/jem.167.3.832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boehm U., Klamp T., Groot M., Howard J. C. Cellular responses to interferon-gamma. Annu Rev Immunol. 1997;15:749–795. doi: 10.1146/annurev.immunol.15.1.749. [DOI] [PubMed] [Google Scholar]
- Boissier M. C., Chiocchia G., Bessis N., Hajnal J., Garotta G., Nicoletti F., Fournier C. Biphasic effect of interferon-gamma in murine collagen-induced arthritis. Eur J Immunol. 1995 May;25(5):1184–1190. doi: 10.1002/eji.1830250508. [DOI] [PubMed] [Google Scholar]
- Butler D. M., Malfait A. M., Mason L. J., Warden P. J., Kollias G., Maini R. N., Feldmann M., Brennan F. M. DBA/1 mice expressing the human TNF-alpha transgene develop a severe, erosive arthritis: characterization of the cytokine cascade and cellular composition. J Immunol. 1997 Sep 15;159(6):2867–2876. [PubMed] [Google Scholar]
- Chu C. Q., Wittmer S., Dalton D. K. Failure to suppress the expansion of the activated CD4 T cell population in interferon gamma-deficient mice leads to exacerbation of experimental autoimmune encephalomyelitis. J Exp Med. 2000 Jul 3;192(1):123–128. doi: 10.1084/jem.192.1.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper S. M., Sriram S., Ranges G. E. Suppression of murine collagen-induced arthritis with monoclonal anti-Ia antibodies and augmentation with IFN-gamma. J Immunol. 1988 Sep 15;141(6):1958–1962. [PubMed] [Google Scholar]
- Dalton D. K., Haynes L., Chu C. Q., Swain S. L., Wittmer S. Interferon gamma eliminates responding CD4 T cells during mycobacterial infection by inducing apoptosis of activated CD4 T cells. J Exp Med. 2000 Jul 3;192(1):117–122. doi: 10.1084/jem.192.1.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- David C. S. Genes for MHC, TCR and MIs determine susceptibility to collagen induced arthritis. APMIS. 1990 Jul;98(7):575–584. doi: 10.1111/j.1699-0463.1990.tb04974.x. [DOI] [PubMed] [Google Scholar]
- Graham M. B., Dalton D. K., Giltinan D., Braciale V. L., Stewart T. A., Braciale T. J. Response to influenza infection in mice with a targeted disruption in the interferon gamma gene. J Exp Med. 1993 Nov 1;178(5):1725–1732. doi: 10.1084/jem.178.5.1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffiths M. M., Cole B. C., Ito J., Harper D. S., Anderson G. D., Cannon G. W., Luthra H. S., David C. S. T-cell receptors and collagen induced arthritis in H-2r mice. Autoimmunity. 1993;14(3):221–229. doi: 10.3109/08916939309077369. [DOI] [PubMed] [Google Scholar]
- Guedez Y. B., Whittington K. B., Clayton J. L., Joosten L. A., van de Loo F. A., van den Berg W. B., Rosloniec E. F. Genetic ablation of interferon-gamma up-regulates interleukin-1beta expression and enables the elicitation of collagen-induced arthritis in a nonsusceptible mouse strain. Arthritis Rheum. 2001 Oct;44(10):2413–2424. doi: 10.1002/1529-0131(200110)44:10<2413::aid-art406>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- Holmdahl R., Jansson L., Gullberg D., Rubin K., Forsberg P. O., Klareskog L. Incidence of arthritis and autoreactivity of anti-collagen antibodies after immunization of DBA/1 mice with heterologous and autologous collagen II. Clin Exp Immunol. 1985 Dec;62(3):639–646. [PMC free article] [PubMed] [Google Scholar]
- Manoury-Schwartz B., Chiocchia G., Bessis N., Abehsira-Amar O., Batteux F., Muller S., Huang S., Boissier M. C., Fournier C. High susceptibility to collagen-induced arthritis in mice lacking IFN-gamma receptors. J Immunol. 1997 Jun 1;158(11):5501–5506. [PubMed] [Google Scholar]
- Matthys P., Hatse S., Vermeire K., Wuyts A., Bridger G., Henson G. W., De Clercq E., Billiau A., Schols D. AMD3100, a potent and specific antagonist of the stromal cell-derived factor-1 chemokine receptor CXCR4, inhibits autoimmune joint inflammation in IFN-gamma receptor-deficient mice. J Immunol. 2001 Oct 15;167(8):4686–4692. doi: 10.4049/jimmunol.167.8.4686. [DOI] [PubMed] [Google Scholar]
- Matthys P., Vermeire K., Mitera T., Heremans H., Huang S., Schols D., De Wolf-Peeters C., Billiau A. Enhanced autoimmune arthritis in IFN-gamma receptor-deficient mice is conditioned by mycobacteria in Freund's adjuvant and by increased expansion of Mac-1+ myeloid cells. J Immunol. 1999 Sep 15;163(6):3503–3510. [PubMed] [Google Scholar]
- Myers L. K., Miyahara H., Terato K., Seyer J. M., Stuart J. M., Kang A. H. Collagen-induced arthritis in B10.RIII mice (H-2r): identification of an arthritogenic T-cell determinant. Immunology. 1995 Apr;84(4):509–513. [PMC free article] [PubMed] [Google Scholar]
- Myers L. K., Seyer J. M., Stuart J. M., Terato K., David C. S., Kang A. H. T cell epitopes of type II collagen that regulate murine collagen-induced arthritis. J Immunol. 1993 Jul 1;151(1):500–505. [PubMed] [Google Scholar]
- Myers L. K., Terato K., Seyer J. M., Stuart J. M., Kang A. H. Characterization of a tolerogenic T cell epitope of type II collagen and its relevance to collagen-induced arthritis. J Immunol. 1992 Aug 15;149(4):1439–1443. [PubMed] [Google Scholar]
- Nabozny G. H., Bull M. J., Hanson J., Griffiths M. M., Luthra H. S., David C. S. Collagen-induced arthritis in T cell receptor V beta congenic B10.Q mice. J Exp Med. 1994 Aug 1;180(2):517–524. doi: 10.1084/jem.180.2.517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakajima H., Hiyama Y., Takamori H., Tsukada W. Cell-mediated transfer of collagen-induced arthritis in mice and its application to the analysis of the inhibitory effects of interferon-gamma and cyclophosphamide. Clin Exp Immunol. 1993 May;92(2):328–335. doi: 10.1111/j.1365-2249.1993.tb03400.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortmann R. A., Shevach E. M. Susceptibility to collagen-induced arthritis: cytokine-mediated regulation. Clin Immunol. 2001 Jan;98(1):109–118. doi: 10.1006/clim.2000.4961. [DOI] [PubMed] [Google Scholar]
- Ranges G. E., Sriram S., Cooper S. M. Prevention of type II collagen-induced arthritis by in vivo treatment with anti-L3T4. J Exp Med. 1985 Sep 1;162(3):1105–1110. doi: 10.1084/jem.162.3.1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saggioro D., Rosato A., Esposito G., Rosenberg M. P., Harrison J., Felber B. K., Pavlakis G. N., Chieco-Bianchi L. Inflammatory polyarthropathy and bone remodeling in HTLV-I Tax-transgenic mice. J Acquir Immune Defic Syndr Hum Retrovirol. 1997 Mar 1;14(3):272–280. doi: 10.1097/00042560-199703010-00012. [DOI] [PubMed] [Google Scholar]
- Staines N. A., Wooley P. H. Collagen arthritis--what can it teach us? Br J Rheumatol. 1994 Sep;33(9):798–807. doi: 10.1093/rheumatology/33.9.798. [DOI] [PubMed] [Google Scholar]
- Vermeire K., Heremans H., Vandeputte M., Huang S., Billiau A., Matthys P. Accelerated collagen-induced arthritis in IFN-gamma receptor-deficient mice. J Immunol. 1997 Jun 1;158(11):5507–5513. [PubMed] [Google Scholar]
- Watson W. C., Townes A. S. Genetic susceptibility to murine collagen II autoimmune arthritis. Proposed relationship to the IgG2 autoantibody subclass response, complement C5, major histocompatibility complex (MHC) and non-MHC loci. J Exp Med. 1985 Dec 1;162(6):1878–1891. doi: 10.1084/jem.162.6.1878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams R. O., Williams D. G., Feldmann M., Maini R. N. Increased limb involvement in murine collagen-induced arthritis following treatment with anti-interferon-gamma. Clin Exp Immunol. 1993 May;92(2):323–327. doi: 10.1111/j.1365-2249.1993.tb03399.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wooley P. H., Cingel B. Staphylococcal enterotoxin B increases the severity of type II collagen induced arthritis in mice. Ann Rheum Dis. 1995 Apr;54(4):298–304. doi: 10.1136/ard.54.4.298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wooley P. H., Luthra H. S., Krco C. J., Stuart J. M., David C. S. Type II collagen-induced arthritis in mice. II. Passive transfer and suppression by intravenous injection of anti-type II collagen antibody or free native type II collagen. Arthritis Rheum. 1984 Sep;27(9):1010–1017. doi: 10.1002/art.1780270907. [DOI] [PubMed] [Google Scholar]
- Wooley P. H., Luthra H. S., Stuart J. M., David C. S. Type II collagen-induced arthritis in mice. I. Major histocompatibility complex (I region) linkage and antibody correlates. J Exp Med. 1981 Sep 1;154(3):688–700. doi: 10.1084/jem.154.3.688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wooley P. H., Sud S., Whalen J. D., Nasser S. Pristane-induced arthritis in mice. V. Susceptibility to pristane-induced arthritis is determined by the genetic regulation of the T cell repertoire. Arthritis Rheum. 1998 Nov;41(11):2022–2031. doi: 10.1002/1529-0131(199811)41:11<2022::AID-ART18>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]