Abstract
Objective: To investigate the efficacy, safety, and dose response of three doses of ibandronate, given intermittently by intravenous (IV) injection every three months, in preventing postmenopausal osteoporosis.
Patients and methods: 629 postmenopausal women, categorised according to time since menopause and baseline lumbar spine (L1–4) bone mineral density (BMD), were enrolled into a multicentre, double blind, placebo controlled trial. They were randomly allocated to receive IV ibandronate 0.5 mg, 1 mg or 2 mg, or placebo every three months. All women received daily calcium supplementation.
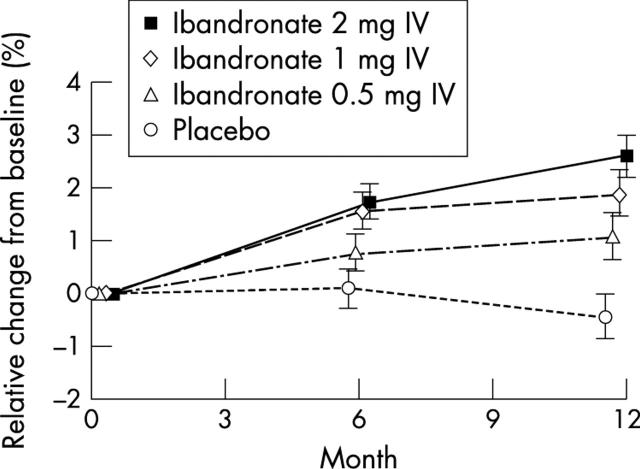
Results: One year's treatment with intermittent IV ibandronate injections produced a dose dependent gain in mean (SD) lumbar spine BMD from baseline of 2.5 (2.5)%, 1.8 (2.6)%, and 1.0 (2.8)% in the groups receiving 2 mg, 1 mg, and 0.5 mg ibandronate, respectively, compared with a loss of BMD of 0.4 (2.4)% in the women in the placebo group; p=0.0001 for each ibandronate dose v placebo. Highest BMD gains occurred in women with osteopenia receiving 2 mg ibandronate. Similarly, at the hip, all three doses of ibandronate produced significantly better gains in BMD than placebo (p<0.05), with the greatest gains in the women with osteopenia receiving the 2 mg dose. Ibandronate concomitantly and dose dependently suppressed markers of bone turnover in comparison with placebo, and injections were well tolerated.
Conclusion: IV ibandronate injections, given every three months, may be an effective alternative to oral bisphosphonates and hormonal therapy in the prevention of bone loss in postmenopausal women.
Full Text
The Full Text of this article is available as a PDF (233.7 KB).
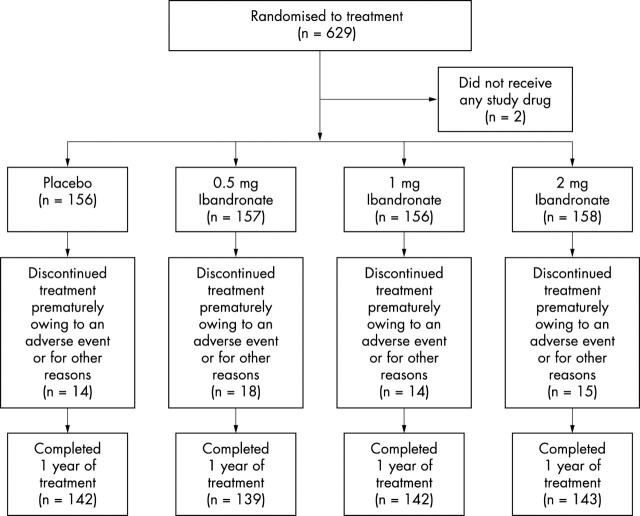
Figure 1.
Patient grouping.
Figure 2.
Relative percentage change from baseline in BMD at the lumbar spine (L1–4) in the ITT population.
Figure 3.
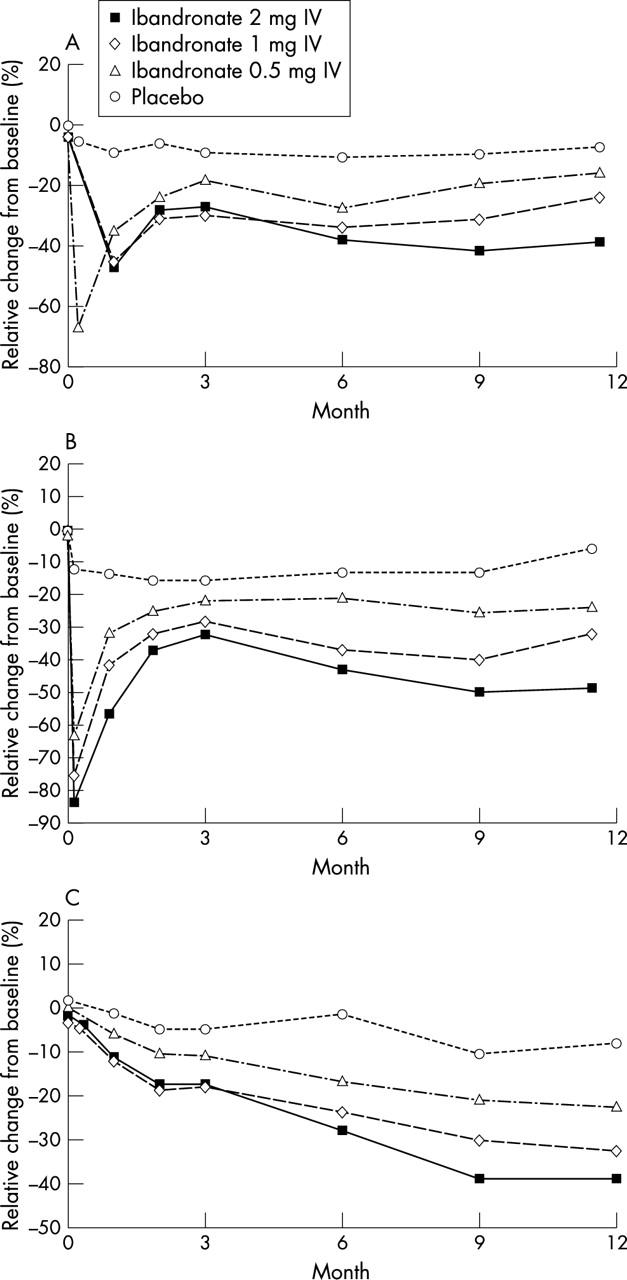
Relative percentage change from baseline in (A) serum CTX, (B) urinary CTX/creatinine, and (C) serum osteocalcin levels in the ITT population.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adami S., Zamberlan N. Adverse effects of bisphosphonates. A comparative review. Drug Saf. 1996 Mar;14(3):158–170. doi: 10.2165/00002018-199614030-00003. [DOI] [PubMed] [Google Scholar]
- Barrett-Connor E. The economic and human costs of osteoporotic fracture. Am J Med. 1995 Feb 27;98(2A):3S–8S. doi: 10.1016/s0002-9343(05)80037-3. [DOI] [PubMed] [Google Scholar]
- Berenson J. R. Zoledronic acid in cancer patients with bone metastases: results of Phase I and II trials. Semin Oncol. 2001 Apr;28(2 Suppl 6):25–34. doi: 10.1016/s0093-7754(01)90262-3. [DOI] [PubMed] [Google Scholar]
- Black D. M., Cummings S. R., Melton L. J., 3rd Appendicular bone mineral and a woman's lifetime risk of hip fracture. J Bone Miner Res. 1992 Jun;7(6):639–646. doi: 10.1002/jbmr.5650070608. [DOI] [PubMed] [Google Scholar]
- Body J. J. Dosing regimens and main adverse events of bisphosphonates. Semin Oncol. 2001 Aug;28(4 Suppl 11):49–53. doi: 10.1016/s0093-7754(01)90232-5. [DOI] [PubMed] [Google Scholar]
- Cummings Steven R., Melton L. Joseph. Epidemiology and outcomes of osteoporotic fractures. Lancet. 2002 May 18;359(9319):1761–1767. doi: 10.1016/S0140-6736(02)08657-9. [DOI] [PubMed] [Google Scholar]
- Ettinger B., Black D. M., Mitlak B. H., Knickerbocker R. K., Nickelsen T., Genant H. K., Christiansen C., Delmas P. D., Zanchetta J. R., Stakkestad J. Reduction of vertebral fracture risk in postmenopausal women with osteoporosis treated with raloxifene: results from a 3-year randomized clinical trial. Multiple Outcomes of Raloxifene Evaluation (MORE) Investigators. JAMA. 1999 Aug 18;282(7):637–645. doi: 10.1001/jama.282.7.637. [DOI] [PubMed] [Google Scholar]
- Fleisch H. Bisphosphonates. Pharmacology and use in the treatment of tumour-induced hypercalcaemic and metastatic bone disease. Drugs. 1991 Dec;42(6):919–944. doi: 10.2165/00003495-199142060-00003. [DOI] [PubMed] [Google Scholar]
- Gatti D., Adami S. New bisphosphonates in the treatment of bone diseases. Drugs Aging. 1999 Oct;15(4):285–296. doi: 10.2165/00002512-199915040-00004. [DOI] [PubMed] [Google Scholar]
- Hosking D., Chilvers C. E., Christiansen C., Ravn P., Wasnich R., Ross P., McClung M., Balske A., Thompson D., Daley M. Prevention of bone loss with alendronate in postmenopausal women under 60 years of age. Early Postmenopausal Intervention Cohort Study Group. N Engl J Med. 1998 Feb 19;338(8):485–492. doi: 10.1056/NEJM199802193380801. [DOI] [PubMed] [Google Scholar]
- Kanis J. A., Delmas P., Burckhardt P., Cooper C., Torgerson D. Guidelines for diagnosis and management of osteoporosis. The European Foundation for Osteoporosis and Bone Disease. Osteoporos Int. 1997;7(4):390–406. doi: 10.1007/BF01623782. [DOI] [PubMed] [Google Scholar]
- Mortensen L., Charles P., Bekker P. J., Digennaro J., Johnston C. C., Jr Risedronate increases bone mass in an early postmenopausal population: two years of treatment plus one year of follow-up. J Clin Endocrinol Metab. 1998 Feb;83(2):396–402. doi: 10.1210/jcem.83.2.4586. [DOI] [PubMed] [Google Scholar]
- Pecherstorfer M., Ludwig H., Schlosser K., Buck S., Huss H. J., Body J. J. Administration of the bisphosphonate ibandronate (BM 21.0955) by intravenous bolus injection. J Bone Miner Res. 1996 May;11(5):587–593. doi: 10.1002/jbmr.5650110506. [DOI] [PubMed] [Google Scholar]
- Ravn P., Clemmesen B., Riis B. J., Christiansen C. The effect on bone mass and bone markers of different doses of ibandronate: a new bisphosphonate for prevention and treatment of postmenopausal osteoporosis: a 1-year, randomized, double-blind, placebo-controlled dose-finding study. Bone. 1996 Nov;19(5):527–533. doi: 10.1016/s8756-3282(96)00229-3. [DOI] [PubMed] [Google Scholar]
- Riggs B. L., Melton L. J., 3rd The prevention and treatment of osteoporosis. N Engl J Med. 1992 Aug 27;327(9):620–627. doi: 10.1056/NEJM199208273270908. [DOI] [PubMed] [Google Scholar]
- Riis B. J., Ise J., von Stein T., Bagger Y., Christiansen C. Ibandronate: a comparison of oral daily dosing versus intermittent dosing in postmenopausal osteoporosis. J Bone Miner Res. 2001 Oct;16(10):1871–1878. doi: 10.1359/jbmr.2001.16.10.1871. [DOI] [PubMed] [Google Scholar]
- Ringe J. D., Dorst A., Faber H., Ibach K., Preuss J. Three-monthly ibandronate bolus injection offers favourable tolerability and sustained efficacy advantage over two years in established corticosteroid-induced osteoporosis. Rheumatology (Oxford) 2003 Apr 16;42(6):743–749. doi: 10.1093/rheumatology/keg205. [DOI] [PubMed] [Google Scholar]
- Russell R. G., Croucher P. I., Rogers M. J. Bisphosphonates: pharmacology, mechanisms of action and clinical uses. Osteoporos Int. 1999;9 (Suppl 2):S66–S80. doi: 10.1007/pl00004164. [DOI] [PubMed] [Google Scholar]
- Schnitzer T., Bone H. G., Crepaldi G., Adami S., McClung M., Kiel D., Felsenberg D., Recker R. R., Tonino R. P., Roux C. Therapeutic equivalence of alendronate 70 mg once-weekly and alendronate 10 mg daily in the treatment of osteoporosis. Alendronate Once-Weekly Study Group. Aging (Milano) 2000 Feb;12(1):1–12. [PubMed] [Google Scholar]
- Simon James A., Lewiecki E. Michael, Smith Mary E., Petruschke Richard A., Wang Lixia, Palmisano Joanne J. Patient preference for once-weekly alendronate 70 mg versus once-daily alendronate 10 mg: a multicenter, randomized, open-label, crossover study. Clin Ther. 2002 Nov;24(11):1871–1886. doi: 10.1016/s0149-2918(02)80085-6. [DOI] [PubMed] [Google Scholar]
- Thiébaud D., Burckhardt P., Kriegbaum H., Huss H., Mulder H., Juttmann J. R., Schöter K. H. Three monthly intravenous injections of ibandronate in the treatment of postmenopausal osteoporosis. Am J Med. 1997 Oct;103(4):298–307. doi: 10.1016/s0002-9343(97)00249-0. [DOI] [PubMed] [Google Scholar]
- Zojer N., Keck A. V., Pecherstorfer M. Comparative tolerability of drug therapies for hypercalcaemia of malignancy. Drug Saf. 1999 Nov;21(5):389–406. doi: 10.2165/00002018-199921050-00004. [DOI] [PubMed] [Google Scholar]