Abstract
Background: Bacterial cell wall (CW) arthritis develops in susceptible strains of rats after a single intraperitoneal injection of the CW from certain bacterial species, both pathogenic and non-pathogenic. For the development of chronic bacterial CW arthritis, the structure of the bacterial peptidoglycan (PG) has been found to be decisive.
Objective: To define the role of PG subtypes in the pathogenesis of chronic bacterial CW arthritis.
Method: Arthritis was induced with CWs of Lactobacillus plantarum, L casei B, L casei C, and L fermentum. Gas chromatography-mass spectrometry was used to measure the presence of CW derived muramic acid in the liver and to determine PG subtypes. CWs were also tested for their resistance to lysozyme in vitro.
Results: These results and those published previously indicate that PGs of CWs which induce chronic arthritis, no matter whether they were derived from strains of Streptococcus, Bifidobacterium, Collinsella, or Lactobacillus, all have lysine as the third amino acid of the PG stem peptide, representing PG subtypes A3α and A4α. Those strains which induce only transient acute arthritis or no arthritis at all do not have lysine in this position, resulting in different PG subtypes.
Conclusions: In vivo degradation of only those PGs with the subtypes A3α and A4α leads to the occurrence of large CW fragments, which persist in tissue and have good proinflammatory ability. CWs with other PG subtypes, even if they are lysozyme resistant, do not cause chronic arthritis, because the released fragments are not phlogistic. It is emphasised that a variety of microbial components not causing inflammation have been found in animal and human synovial tissue.
Full Text
The Full Text of this article is available as a PDF (241.5 KB).
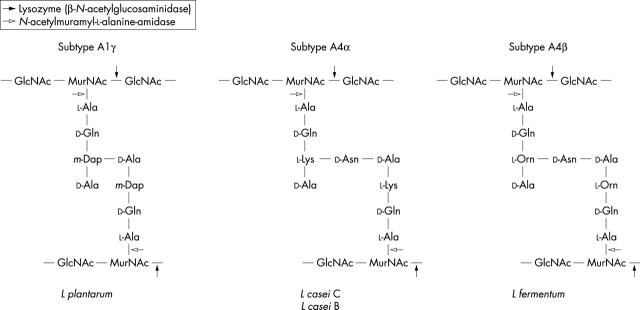
Figure 1.
PG structures in the Lactobacillus strains used. The L plantarum belongs to the D-glutamine-m-diaminopimelic acid-D-alanine subtype (A1γ), the two L casei share the L-lysine-D-asparagine subtype (A4α), whereas L fermentum has the subtype L-ornithine-D-asparagine (A4ß). The enzymes degrading PG cleave specific bonds: lysozyme (ß-N-acetylglucosaminidase) and N-acetylmuramyl-L-alanine-amidase.
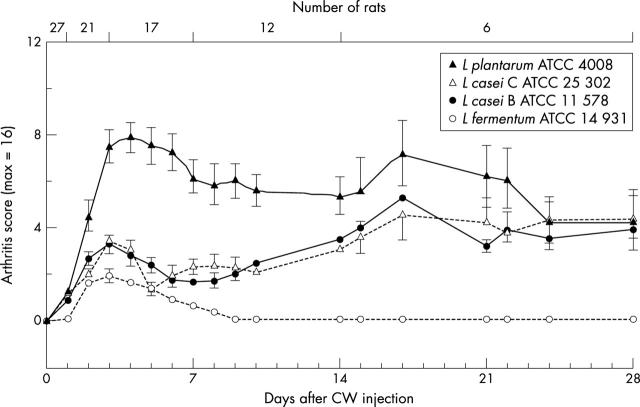
Figure 2.
Development of arthritis in rats injected IP with Lactobacillus CW. The injection dose was 24 mg of CW dry weight/100 g of rat body weight for L plantarum ATCC 4008 or L casei C ATCC 25302, and 8 mg for L casei B ATCC 11578 or L fermentum ATCC 14931. The arthritis score is calculated as a mean value (SEM) for the number of rats indicated at the top. Rats which died on day 2 are excluded from this figure.
Figure 3.
Muramic acid content in rat liver analysed by GC-MS. On day 0 rats were injected IP with CW from L plantarum ATCC 4008, L casei C ATCC 25302, L casei B ATCC 11578, or L fermentum ATCC 14931. The muramic acid levels are given as the mean value (SEM) for four to six rats. Asterisks indicate significant differences (p<0.05) when the rats injected with L plantarum, L casei C or L casei B CWs were compared with those injected with L fermentum CW. Significance is shown separately for each day of analysis.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderle S. K., Allen J. B., Wilder R. L., Eisenberg R. A., Cromartie W. J., Schwab J. H. Measurement of streptococcal cell wall in tissues of rats resistant or susceptible to cell wall-induced chronic erosive arthritis. Infect Immun. 1985 Sep;49(3):836–837. doi: 10.1128/iai.49.3.836-837.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burroughs M., Rozdzinski E., Geelen S., Tuomanen E. A structure-activity relationship for induction of meningeal inflammation by muramyl peptides. J Clin Invest. 1993 Jul;92(1):297–302. doi: 10.1172/JCI116565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang Y. H., Pearson C. M., Chedid L. Adjuvant polyarthritis. V. Induction by N-acetylmuramyl-L-alanyl-D-isoglutamine, the smallest peptide subunit of bacterial peptidoglycan. J Exp Med. 1981 Apr 1;153(4):1021–1026. doi: 10.1084/jem.153.4.1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen T., Isomäki P., Rimpiläinen M., Toivanen P. Human cytokine responses induced by gram-positive cell walls of normal intestinal microbiota. Clin Exp Immunol. 1999 Nov;118(2):261–267. doi: 10.1046/j.1365-2249.1999.01047.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cromartie W. J., Craddock J. G., Schwab J. H., Anderle S. K., Yang C. H. Arthritis in rats after systemic injection of streptococcal cells or cell walls. J Exp Med. 1977 Dec 1;146(6):1585–1602. doi: 10.1084/jem.146.6.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberg R., Fox A., Greenblatt J. J., Anderle S. K., Cromartie W. J., Schwab J. H. Measurement of bacterial cell wall in tissues by solid-phase radioimmunoassay: correlation of distribution and persistence with experimental arthritis in rats. Infect Immun. 1982 Oct;38(1):127–135. doi: 10.1128/iai.38.1.127-135.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox A., Brown R. R., Anderle S. K., Chetty C., Cromartie W. J., Gooder H., Schwab J. H. Arthropathic properties related to the molecular weight of peptidoglycan-polysaccharide polymers of streptococcal cell walls. Infect Immun. 1982 Mar;35(3):1003–1010. doi: 10.1128/iai.35.3.1003-1010.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbart J., Harrison J., Parks C., Fox A. Analysis of the amino acid and sugar composition of streptococcal cell walls by gas chromatography-mass spectrometry. J Chromatogr. 1988 Jun 10;441(2):323–333. doi: 10.1016/s0021-9673(01)83875-9. [DOI] [PubMed] [Google Scholar]
- Gripenberg-Lerche C., Skurnik M., Toivanen P. Role of YadA-mediated collagen binding in arthritogenicity of Yersinia enterocolitica serotype O:8: experimental studies with rats. Infect Immun. 1995 Aug;63(8):3222–3226. doi: 10.1128/iai.63.8.3222-3226.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guinand M., Ghuysen J. M., Schleifer K. H., Kandler O. The peptidoglycan in walls of Butyribacterium rettgeri. Biochemistry. 1969 Jan;8(1):200–207. doi: 10.1021/bi00829a029. [DOI] [PubMed] [Google Scholar]
- Hall E. A., Knox K. W. Properties of the polysaccharide and mucopeptide components of the cell wall of Lactobacillus casei. Biochem J. 1965 Aug;96(2):310–318. doi: 10.1042/bj0960310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heumann D., Barras C., Severin A., Glauser M. P., Tomasz A. Gram-positive cell walls stimulate synthesis of tumor necrosis factor alpha and interleukin-6 by human monocytes. Infect Immun. 1994 Jul;62(7):2715–2721. doi: 10.1128/iai.62.7.2715-2721.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hungerer K. D., Fleck J., Tipper D. J. Structure of the cell wall peptidoglycan of Lactobacillus casei RO94. Biochemistry. 1969 Sep;8(9):3567–3577. doi: 10.1021/bi00837a012. [DOI] [PubMed] [Google Scholar]
- Kageyama A., Benno Y., Nakase T. Phylogenetic and phenotypic evidence for the transfer of Eubacterium aerofaciens to the genus Collinsella as Collinsella aerofaciens gen. nov., comb. nov. Int J Syst Bacteriol. 1999 Apr;49(Pt 2):557–565. doi: 10.1099/00207713-49-2-557. [DOI] [PubMed] [Google Scholar]
- Kawasaki A., Takada H., Kotani S., Inai S., Nagaki K., Matsumoto M., Yokogawa K., Kawata S., Kusumoto S., Shiba T. Activation of the human complement cascade by bacterial cell walls, peptidoglycans, water-soluble peptidoglycan components, and synthetic muramylpeptides--studies on active components and structural requirements. Microbiol Immunol. 1987;31(6):551–569. doi: 10.1111/j.1348-0421.1987.tb03117.x. [DOI] [PubMed] [Google Scholar]
- Kohashi O., Pearson C. M., Watanabe Y., Kotani S., Koga T. Structural requirements for arthritogenicity of peptidoglycans from Staphylococcus aureus and Lactobacillus plant arum and analogous synthetic compounds. J Immunol. 1976 Jun;116(6):1635–1639. [PubMed] [Google Scholar]
- Kool J., Gerrits-Boeye M. Y., Severijnen A. J., Hazenberg M. P. Immunohistology of joint inflammation induced in rats by cell wall fragments of Eubacterium aerofaciens. Scand J Immunol. 1992 Sep;36(3):497–506. doi: 10.1111/j.1365-3083.1992.tb02965.x. [DOI] [PubMed] [Google Scholar]
- Kotani S., Tsujimoto M., Koga T., Nagao S., Tanaka A., Kawata S. Chemical structure and biological activity relationship of bacterial cell walls and muramyl peptides. Fed Proc. 1986 Oct;45(11):2534–2540. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lehman T. J., Allen J. B., Plotz P. H., Wilder R. L. Bacterial cell wall composition, lysozyme resistance, and the induction of chronic arthritis in rats. Rheumatol Int. 1985;5(4):163–167. doi: 10.1007/BF00541517. [DOI] [PubMed] [Google Scholar]
- Lehman T. J., Allen J. B., Plotz P. H., Wilder R. L. Lactobacillus casei cell wall-induced arthritis in rats: cell wall fragment distribution and persistence in chronic arthritis-susceptible LEW/N and -resistant F344/N rats. Arthritis Rheum. 1984 Aug;27(8):939–942. doi: 10.1002/art.1780270815. [DOI] [PubMed] [Google Scholar]
- Lehman T. J., Allen J. B., Plotz P. H., Wilder R. L. Polyarthritis in rats following the systemic injection of Lactobacillus casei cell walls in aqueous suspension. Arthritis Rheum. 1983 Oct;26(10):1259–1265. doi: 10.1002/art.1780261013. [DOI] [PubMed] [Google Scholar]
- Lehtonen L., Eerola E., Oksman P., Toivanen P. Muramic acid in peripheral blood leukocytes of healthy human subjects. J Infect Dis. 1995 Apr;171(4):1060–1064. doi: 10.1093/infdis/171.4.1060. [DOI] [PubMed] [Google Scholar]
- Majcherczyk P. A., Langen H., Heumann D., Fountoulakis M., Glauser M. P., Moreillon P. Digestion of Streptococcus pneumoniae cell walls with its major peptidoglycan hydrolase releases branched stem peptides carrying proinflammatory activity. J Biol Chem. 1999 Apr 30;274(18):12537–12543. doi: 10.1074/jbc.274.18.12537. [DOI] [PubMed] [Google Scholar]
- Martin S. A., Karnovsky M. L., Krueger J. M., Pappenheimer J. R., Biemann K. Peptidoglycans as promoters of slow-wave sleep. I. Structure of the sleep-promoting factor isolated from human urine. J Biol Chem. 1984 Oct 25;259(20):12652–12658. [PubMed] [Google Scholar]
- Martínez-Martínez L., Timmerman C. P., Fleer A., Verhoef J. Chemiluminescence of human polymorphonuclear leucocytes after stimulation with whole cells and cell-wall components of Staphylococcus epidermidis. J Med Microbiol. 1993 Sep;39(3):196–203. doi: 10.1099/00222615-39-3-196. [DOI] [PubMed] [Google Scholar]
- Okitsu-Negishi S., Nakano I., Suzuki K., Hashira S., Abe T., Yoshino K. The induction of cardioangitis by Lactobacillus casei cell wall in mice. I. The cytokine production from murine macrophages by Lactobacillus casei cell wall extract. Clin Immunol Immunopathol. 1996 Jan;78(1):30–40. doi: 10.1006/clin.1996.0005. [DOI] [PubMed] [Google Scholar]
- Sartor R. B., Cromartie W. J., Powell D. W., Schwab J. H. Granulomatous enterocolitis induced in rats by purified bacterial cell wall fragments. Gastroenterology. 1985 Sep;89(3):587–595. doi: 10.1016/0016-5085(85)90455-x. [DOI] [PubMed] [Google Scholar]
- Sato K., Saito H., Tomioka H., Yokokura T. Enhancement of host resistance against Listeria infection by Lactobacillus casei: efficacy of cell wall preparation of Lactobacillus casei. Microbiol Immunol. 1988;32(12):1189–1200. doi: 10.1111/j.1348-0421.1988.tb01483.x. [DOI] [PubMed] [Google Scholar]
- Schleifer K. H., Kandler O. Peptidoglycan types of bacterial cell walls and their taxonomic implications. Bacteriol Rev. 1972 Dec;36(4):407–477. doi: 10.1128/br.36.4.407-477.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schumacher H. R., Jr, Arayssi T., Crane M., Lee J., Gerard H., Hudson A. P., Klippel J. Chlamydia trachomatis nucleic acids can be found in the synovium of some asymptomatic subjects. Arthritis Rheum. 1999 Jun;42(6):1281–1284. doi: 10.1002/1529-0131(199906)42:6<1281::AID-ANR27>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- Schwab J. H. Phlogistic properties of peptidoglycan-polysaccharide polymers from cell walls of pathogenic and normal-flora bacteria which colonize humans. Infect Immun. 1993 Nov;61(11):4535–4539. doi: 10.1128/iai.61.11.4535-4539.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Severijnen A. J., van Kleef R., Hazenberg M. P., van de Merwe J. P. Cell wall fragments from major residents of the human intestinal flora induce chronic arthritis in rats. J Rheumatol. 1989 Aug;16(8):1061–1068. [PubMed] [Google Scholar]
- Severijnen A. J., van Kleef R., Hazenberg M. P., van de Merwe J. P. Chronic arthritis induced in rats by cell wall fragments of Eubacterium species from the human intestinal flora. Infect Immun. 1990 Feb;58(2):523–528. doi: 10.1128/iai.58.2.523-528.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Severin A. I., Kokeguchi S., Kato K. Chemical composition of Eubacterium alactolyticum cell wall peptidoglycan. Arch Microbiol. 1989;151(4):348–352. doi: 10.1007/BF00406563. [DOI] [PubMed] [Google Scholar]
- Simelyte E., Isomäki P., Rimpiläinen M., Zhang X., Toivanen P. Cytokine production in arthritis susceptible and resistant rats: a study with arthritogenic and non-arthritogenic Lactobacillus cell walls. Scand J Immunol. 2001 Feb;53(2):132–138. doi: 10.1046/j.1365-3083.2001.00846.x. [DOI] [PubMed] [Google Scholar]
- Simelyte E., Rimpiläinen M., Lehtonen L., Zhang X., Toivanen P. Bacterial cell wall-induced arthritis: chemical composition and tissue distribution of four Lactobacillus strains. Infect Immun. 2000 Jun;68(6):3535–3540. doi: 10.1128/iai.68.6.3535-3540.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simelyte E., Rimpiläinen M., Rantakokko K., Lehtonen L., Zhang X., Aho H., Isomäki P., Toivanen P. Tissue distribution and persistence of arthritogenic and non-arthritogenic Eubacterium cell walls. Clin Exp Rheumatol. 1999 May-Jun;17(3):281–288. [PubMed] [Google Scholar]
- Stewart-Tull D. E. The immunological activities of bacterial peptidoglycans. Annu Rev Microbiol. 1980;34:311–340. doi: 10.1146/annurev.mi.34.100180.001523. [DOI] [PubMed] [Google Scholar]
- Stimpson S. A., Brown R. R., Anderle S. K., Klapper D. G., Clark R. L., Cromartie W. J., Schwab J. H. Arthropathic properties of cell wall polymers from normal flora bacteria. Infect Immun. 1986 Jan;51(1):240–249. doi: 10.1128/iai.51.1.240-249.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stimpson S. A., Esser R. E., Cromartie W. J., Schwab J. H. Comparison of in vivo degradation of 125I-labeled peptidoglycan-polysaccharide fragments from group A and group D streptococci. Infect Immun. 1986 May;52(2):390–396. doi: 10.1128/iai.52.2.390-396.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stimpson S. A., Lerch R. A., Cleland D. R., Yarnall D. P., Clark R. L., Cromartie W. J., Schwab J. H. Effect of acetylation on arthropathic activity of group A streptococcal peptidoglycan-polysaccharide fragments. Infect Immun. 1987 Jan;55(1):16–23. doi: 10.1128/iai.55.1.16-23.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Söderlund M., von Essen R., Haapasaari J., Kiistala U., Kiviluoto O., Hedman K. Persistence of parvovirus B19 DNA in synovial membranes of young patients with and without chronic arthropathy. Lancet. 1997 Apr 12;349(9058):1063–1065. doi: 10.1016/S0140-6736(96)09110-6. [DOI] [PubMed] [Google Scholar]
- Tomasz A., Saukkonen K. The nature of cell wall-derived inflammatory components of pneumococci. Pediatr Infect Dis J. 1989 Dec;8(12):902–903. doi: 10.1097/00006454-198912000-00034. [DOI] [PubMed] [Google Scholar]
- Tuomanen E., Liu H., Hengstler B., Zak O., Tomasz A. The induction of meningeal inflammation by components of the pneumococcal cell wall. J Infect Dis. 1985 May;151(5):859–868. doi: 10.1093/infdis/151.5.859. [DOI] [PubMed] [Google Scholar]
- Wahl S. M., Hunt D. A., Allen J. B., Wilder R. L., Paglia L., Hand A. R. Bacterial cell wall-induced hepatic granulomas. An in vivo model of T cell-dependent fibrosis. J Exp Med. 1986 Apr 1;163(4):884–902. doi: 10.1084/jem.163.4.884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilder R. L., Allen J. B., Wahl L. M., Calandra G. B., Wahl S. M. The pathogenesis of group A streptococcal cell wall-induced polyarthritis in the rat. Comparative studies in arthritis resistant and susceptible inbred rat strains. Arthritis Rheum. 1983 Dec;26(12):1442–1451. doi: 10.1002/art.1780261205. [DOI] [PubMed] [Google Scholar]
- Zhang L., Nikkari S., Skurnik M., Ziegler T., Luukkainen R., Möttönen T., Toivanen P. Detection of herpesviruses by polymerase chain reaction in lymphocytes from patients with rheumatoid arthritis. Arthritis Rheum. 1993 Aug;36(8):1080–1086. doi: 10.1002/art.1780360808. [DOI] [PubMed] [Google Scholar]
- Zhang X., Rimpiläinen M., Hoffmann B., Simelyte E., Aho H., Toivanen P. Experimental chronic arthritis and granulomatous inflammation induced by bifidobacterium cell walls. Scand J Immunol. 2001 Jul-Aug;54(1-2):171–179. doi: 10.1046/j.1365-3083.2001.00936.x. [DOI] [PubMed] [Google Scholar]
- Zhang X., Rimpiläinen M., Simelyte E., Toivanen P. Characterisation of Eubacterium cell wall: peptidoglycan structure determines arthritogenicity. Ann Rheum Dis. 2001 Mar;60(3):269–274. doi: 10.1136/ard.60.3.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X., Rimpiläinen M., Simelyte E., Toivanen P. Enzyme degradation and proinflammatory activity in arthritogenic and nonarthritogenic Eubacterium aerofaciens cell walls. Infect Immun. 2001 Dec;69(12):7277–7284. doi: 10.1128/IAI.69.12.7277-7284.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X., Rimpiläinen M., Simelyte E., Toivanen P. What determines arthritogenicity of bacterial cell wall? A study on Eubacterium cell wall-induced arthritis. Rheumatology (Oxford) 2000 Mar;39(3):274–282. doi: 10.1093/rheumatology/39.3.274. [DOI] [PubMed] [Google Scholar]