Abstract
Objective: To determine the survival and clinical effectiveness of leflunomide (LEF) compared with methotrexate (MTX) and sulfasalazine (SSZ) for RA in an observational study.
Methods: An observational database of 1088 patients and 5141 patient years of DMARD treatment (2680 courses) from two academic hospitals was filtered for treatment with LEF, MTX, and SSZ. LEF treatment groups were matched for patients' age, baseline ESR, number of previous DMARDs, and hospital cohort with MTX and SSZ treatment groups. For these treatments, Kaplan-Meier analyses of time until the drug was discontinued (drug "survival"), and the effectiveness and safety of continuation of treatment, were performed. The change in disease activity markers (CRP, ESR) was compared between the groups.
Results: The median dose during the study increased from 10 to 15 mg MTX/week and from 1.5 to 2.0 g SSZ/day. Matched survival analysis showed better retention rates for MTX (mean (SEM) survival 28 (1) months) than for LEF (20 (1) months; p=0.001), whereas retention rates of SSZ (23 (1) months) were similar to those of LEF (p=NS). Treatments were stopped earlier because of adverse events (AEs, 3 months) than because of ineffectiveness (IE, 10 months; p<0.001). LEF and MTX were less likely to be stopped because of AEs than SSZ. LEF courses were stopped earlier for AEs (p<0.001) than MTX.
Conclusions: Current dosing strategies should be re-evaluated, and coping strategies for common AEs should be investigated. This will be necessary to achieve better drug retention of LEF. At present, MTX continues to be the most effective drug in clinical practice.
Full Text
The Full Text of this article is available as a PDF (549.5 KB).
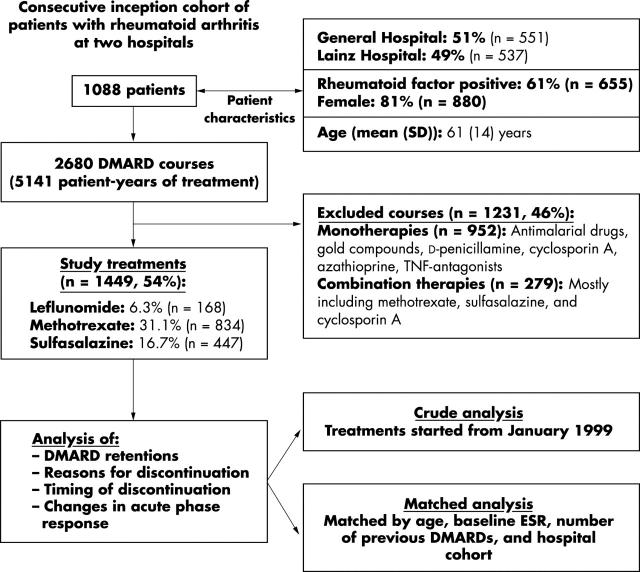
Figure 1.
Population studied, characteristics, and flow chart of analysis. The study group comprised a consecutive inception cohort of 1088 patients with RA, who had undergone 2680 DMARD courses.
Figure 2.
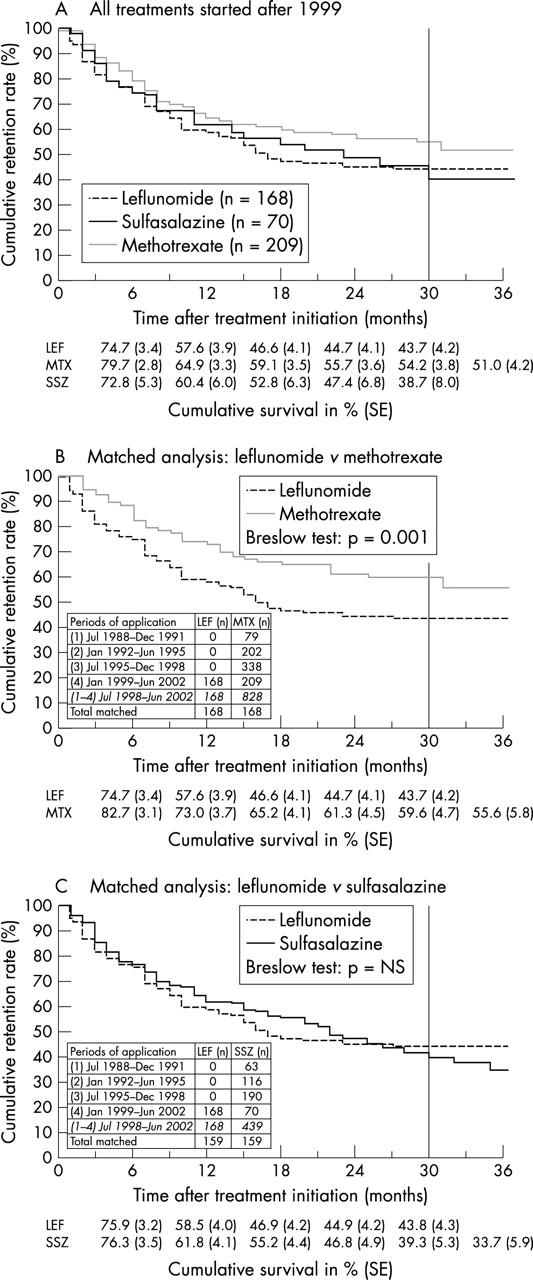
Cumulative drug retentions (%) of LEF, MTX, and SSZ. The period to the right of the reference line (at 30 months) indicates an unduly small number of patients left at risk (<10%). (A) All treatments started after 1999, mean survivals (SE) of the drugs (months)—MTX (n=209): 26 (1); SSZ (n=70): 23 (2); LEF (n=168): 20 (1). Breslow test: p=0.03 for MTX v LEF, otherwise: p=NS. (B) Matched analysis: LEF (n=168) v MTX (n=168)—MTX: 28 (1) months; LEF: 20 (1) months. Breslow test: p=0.001. (C) Matched analysis: LEF (n=159) v SSZ (n=159)—LEF: 20 (1) months; SSZ: 23 (1) months. Breslow test: p=NS.
Figure 3.
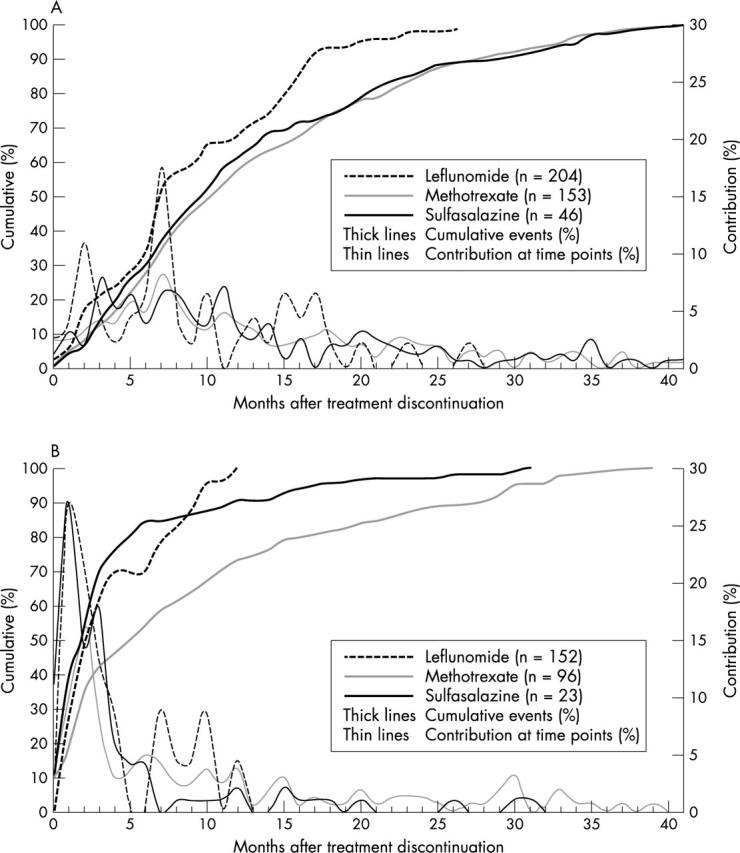
Timing of DMARD discontinuation due to (A) inefficiency (A) and (B) AEs. Only treatments with an event during the first 42 months are displayed (n). The median timing of discontinuations (for all DMARDs) due to inefficiency (A) was 10 months (quartiles: 6; 18 months); and due to adverse events (B) was: median 3 months (1; 10 months) (p<0.001, Kolmogorov-Smirnov test for equality of distributions). Between-drug comparison for timing of inefficiency (A): p=NS; and for timing of adverse events (B): p<0.001 (earlier timing of events while receiving LEF and SSZ than while receiving MTX treatment).
Figure 4.
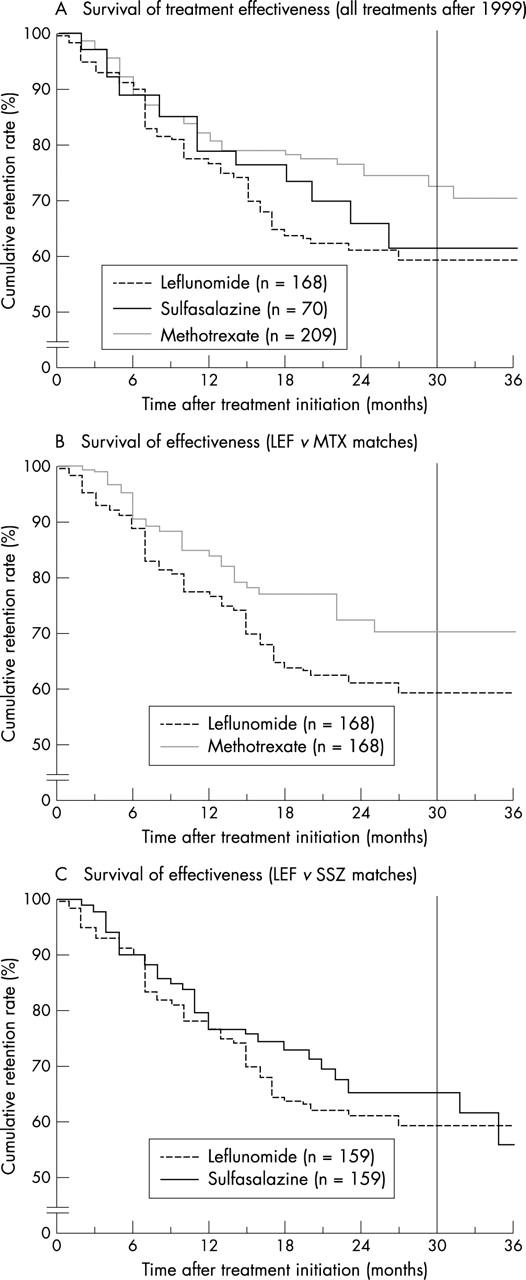
Survival of treatment effectiveness. Cumulative drug retentions (%) of LEF, MTX, and SSZ, when only discontinuation due to inefficiency was analysed, assuming permanent safety otherwise (that is, censoring at time of occurrence). The period to the right of the reference line (30 months) indicates an unduly small number of patients left at risk (<10%). Note the discontinuous y axis. (A) All treatments after 1999, mean survivals (SE) of the drugs (months)—MTX (n=209): 33 (1); SSZ (n=70): 30 (2); and LEF (n=168): 26 (1). Breslow test: p=0.04 for MTX v LEF, otherwise: p=NS. (B) Matched analysis of LEF v MTX (n=168)—LEF: 26 (1) v MTX: 32 (1); Breslow test: p=0.04. (C) Matched analysis of LEF v SSZ (n=159)—LEF: 26 (1) v SSZ: 30 (1); Breslow test: p=NS.
Figure 5.
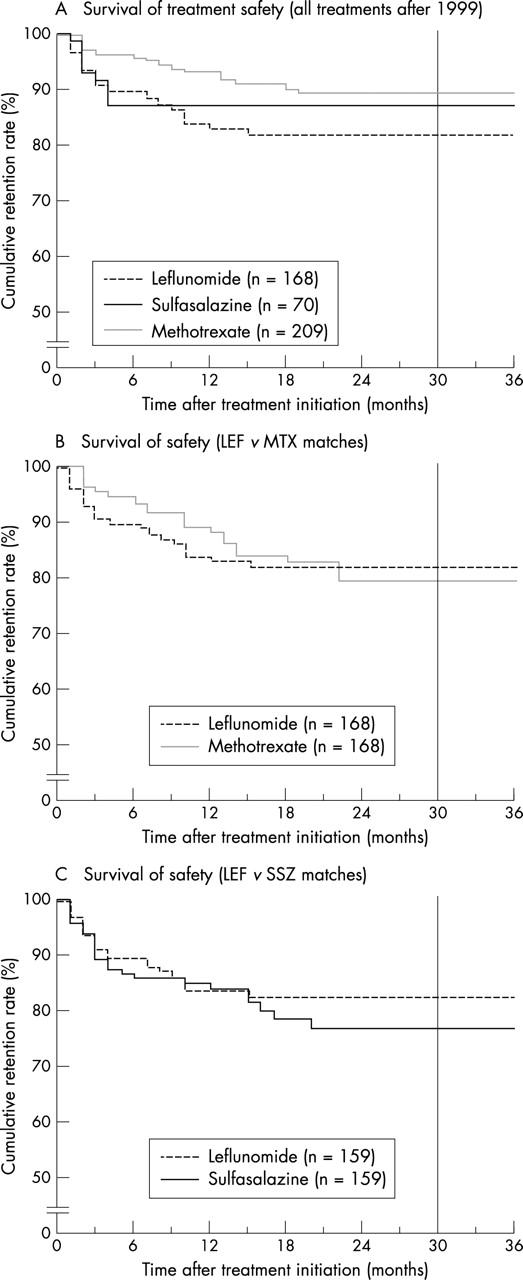
survival of treatment safety. Cumulative drug retentions (%) of LEF, MTX, and SSZ, while only discontinuation due to adverse events was analysed, assuming permanent effectiveness otherwise (that is, censoring at time of occurrence). The period to the right of the reference line (30 months) indicates an unduly small number of patients left at risk (<10%). Note the discontinuous y axis. (A) All treatments after 1999, mean survivals (SE) of the drugs (months)—MTX (n=209): 37 (1); SSZ (n=70): 36 (2); and LEF (n=168): 30 (1). Breslow test: p=0.01 for MTX v LEF, otherwise: p=NS. (B) Matched analysis of LEF v MTX (n=168)—LEF: 30 (1) v MTX: 34 (1); Breslow test: p=NS. (C) Matched analysis of LEF v SSZ (n=159)—LEF: 31 (1) v SSZ: 33 (1); Breslow test: p=NS.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aletaha D., Smolen J. S. The rheumatoid arthritis patient in the clinic: comparing more than 1,300 consecutive DMARD courses. Rheumatology (Oxford) 2002 Dec;41(12):1367–1374. doi: 10.1093/rheumatology/41.12.1367. [DOI] [PubMed] [Google Scholar]
- Aletaha Daniel, Smolen Josef S. Effectiveness profiles and dose dependent retention of traditional disease modifying antirheumatic drugs for rheumatoid arthritis. An observational study. J Rheumatol. 2002 Aug;29(8):1631–1638. [PubMed] [Google Scholar]
- Aletaha Daniel, Smolen Josef S. Laboratory testing in rheumatoid arthritis patients taking disease-modifying antirheumatic drugs: clinical evaluation and cost analysis. Arthritis Rheum. 2002 Apr 15;47(2):181–188. doi: 10.1002/art.10266. [DOI] [PubMed] [Google Scholar]
- Anderson J. J., Wells G., Verhoeven A. C., Felson D. T. Factors predicting response to treatment in rheumatoid arthritis: the importance of disease duration. Arthritis Rheum. 2000 Jan;43(1):22–29. doi: 10.1002/1529-0131(200001)43:1<22::AID-ANR4>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- Arnett F. C., Edworthy S. M., Bloch D. A., McShane D. J., Fries J. F., Cooper N. S., Healey L. A., Kaplan S. R., Liang M. H., Luthra H. S. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum. 1988 Mar;31(3):315–324. doi: 10.1002/art.1780310302. [DOI] [PubMed] [Google Scholar]
- Atkinson J. P. C-reactive protein: a rheumatologist's friend revisited. Arthritis Rheum. 2001 May;44(5):995–996. doi: 10.1002/1529-0131(200105)44:5<995::AID-ANR177>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- Black N. Why we need observational studies to evaluate the effectiveness of health care. BMJ. 1996 May 11;312(7040):1215–1218. doi: 10.1136/bmj.312.7040.1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breedveld F. C., Dayer J. M. Leflunomide: mode of action in the treatment of rheumatoid arthritis. Ann Rheum Dis. 2000 Nov;59(11):841–849. doi: 10.1136/ard.59.11.841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breslow N. E., Edler L., Berger J. A two-sample censored-data rank test for acceleration. Biometrics. 1984 Dec;40(4):1049–1062. [PubMed] [Google Scholar]
- Devlin J., Gough A., Huissoon A., Perkins P., Holder R., Reece R., Arthur V., Emery P. The acute phase and function in early rheumatoid arthritis. C-reactive protein levels correlate with functional outcome. J Rheumatol. 1997 Jan;24(1):9–13. [PubMed] [Google Scholar]
- Ellis S. J., Adams R. F. The cult of the double-blind placebo-controlled trial. Br J Clin Pract. 1997 Jan-Feb;51(1):36–39. [PubMed] [Google Scholar]
- Emery P., Breedveld F. C., Lemmel E. M., Kaltwasser J. P., Dawes P. T., Gömör B., Van Den Bosch F., Nordström D., Bjorneboe O., Dahl R. A comparison of the efficacy and safety of leflunomide and methotrexate for the treatment of rheumatoid arthritis. Rheumatology (Oxford) 2000 Jun;39(6):655–665. doi: 10.1093/rheumatology/39.6.655. [DOI] [PubMed] [Google Scholar]
- Emery P. Therapeutic approaches for early rheumatoid arthritis. How early? How aggressive? Br J Rheumatol. 1995 Nov;34 (Suppl 2):87–90. [PubMed] [Google Scholar]
- Fries J. F. Current treatment paradigms in rheumatoid arthritis. Rheumatology (Oxford) 2000 Jun;39 (Suppl 1):30–35. doi: 10.1093/oxfordjournals.rheumatology.a031492. [DOI] [PubMed] [Google Scholar]
- Fries J. F. Effectiveness and toxicity considerations in outcome directed therapy in rheumatoid arthritis. J Rheumatol Suppl. 1996 Mar;44:102–106. [PubMed] [Google Scholar]
- Furst D. E. Aggressive strategies for treating aggressive rheumatoid arthritis: has the case been proven? Lancet. 2000 Jul 15;356(9225):183–184. doi: 10.1016/S0140-6736(00)02477-6. [DOI] [PubMed] [Google Scholar]
- Hawley D. J., Wolfe F. Are the results of controlled clinical trials and observational studies of second line therapy in rheumatoid arthritis valid and generalizable as measures of rheumatoid arthritis outcome: analysis of 122 studies. J Rheumatol. 1991 Jul;18(7):1008–1014. [PubMed] [Google Scholar]
- Hoffmeister R. T. Methotrexate therapy in rheumatoid arthritis: 15 years experience. Am J Med. 1983 Dec 30;75(6A):69–73. doi: 10.1016/0002-9343(83)90477-1. [DOI] [PubMed] [Google Scholar]
- Hurst Stacey, Kallan Michael J., Wolfe Frederick J., Fries James F., Albert Daniel A. Methotrexate, hydroxychloroquine, and intramuscular gold in rheumatoid arthritis: relative area under the curve effectiveness and sequence effects. J Rheumatol. 2002 Aug;29(8):1639–1645. [PubMed] [Google Scholar]
- Jeurissen M. E., Boerbooms A. M., van de Putte L. B., Doesburg W. H., Lemmens A. M. Influence of methotrexate and azathioprine on radiologic progression in rheumatoid arthritis. A randomized, double-blind study. Ann Intern Med. 1991 Jun 15;114(12):999–1004. doi: 10.7326/0003-4819-114-12-999. [DOI] [PubMed] [Google Scholar]
- Morgan S. L., Baggott J. E., Vaughn W. H., Young P. K., Austin J. V., Krumdieck C. L., Alarcón G. S. The effect of folic acid supplementation on the toxicity of low-dose methotrexate in patients with rheumatoid arthritis. Arthritis Rheum. 1990 Jan;33(1):9–18. doi: 10.1002/art.1780330102. [DOI] [PubMed] [Google Scholar]
- Pincus T., Marcum S. B., Callahan L. F. Longterm drug therapy for rheumatoid arthritis in seven rheumatology private practices: II. Second line drugs and prednisone. J Rheumatol. 1992 Dec;19(12):1885–1894. [PubMed] [Google Scholar]
- Pincus T. Rheumatoid arthritis: disappointing long-term outcomes despite successful short-term clinical trials. J Clin Epidemiol. 1988;41(11):1037–1041. doi: 10.1016/0895-4356(88)90072-8. [DOI] [PubMed] [Google Scholar]
- Pincus T., Stein C. M. Why randomized controlled clinical trials do not depict accurately long-term outcomes in rheumatoid arthritis: some explanations and suggestions for future studies. Clin Exp Rheumatol. 1997 May-Jun;15 (Suppl 17):S27–S38. [PubMed] [Google Scholar]
- Pocock Stuart J., Clayton Tim C., Altman Douglas G. Survival plots of time-to-event outcomes in clinical trials: good practice and pitfalls. Lancet. 2002 May 11;359(9318):1686–1689. doi: 10.1016/S0140-6736(02)08594-X. [DOI] [PubMed] [Google Scholar]
- Pullar T., Hunter J. A., Capell H. A. Sulphasalazine in rheumatoid arthritis: a double blind comparison of sulphasalazine with placebo and sodium aurothiomalate. Br Med J (Clin Res Ed) 1983 Oct 15;287(6399):1102–1104. doi: 10.1136/bmj.287.6399.1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott D. L., Smolen J. S., Kalden J. R., van de Putte L. B., Larsen A., Kvien T. K., Schattenkirchner M., Nash P., Oed C., Loew-Friedrich I. Treatment of active rheumatoid arthritis with leflunomide: two year follow up of a double blind, placebo controlled trial versus sulfasalazine. Ann Rheum Dis. 2001 Oct;60(10):913–923. doi: 10.1136/ard.60.10.913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharp J. T., Strand V., Leung H., Hurley F., Loew-Friedrich I. Treatment with leflunomide slows radiographic progression of rheumatoid arthritis: results from three randomized controlled trials of leflunomide in patients with active rheumatoid arthritis. Leflunomide Rheumatoid Arthritis Investigators Group. Arthritis Rheum. 2000 Mar;43(3):495–505. doi: 10.1002/1529-0131(200003)43:3<495::AID-ANR4>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- Strand V., Cohen S., Schiff M., Weaver A., Fleischmann R., Cannon G., Fox R., Moreland L., Olsen N., Furst D. Treatment of active rheumatoid arthritis with leflunomide compared with placebo and methotrexate. Leflunomide Rheumatoid Arthritis Investigators Group. Arch Intern Med. 1999 Nov 22;159(21):2542–2550. doi: 10.1001/archinte.159.21.2542. [DOI] [PubMed] [Google Scholar]
- Strand V., Tugwell P., Bombardier C., Maetzel A., Crawford B., Dorrier C., Thompson A., Wells G. Function and health-related quality of life: results from a randomized controlled trial of leflunomide versus methotrexate or placebo in patients with active rheumatoid arthritis. Leflunomide Rheumatoid Arthritis Investigators Group. Arthritis Rheum. 1999 Sep;42(9):1870–1878. doi: 10.1002/1529-0131(199909)42:9<1870::AID-ANR11>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- Weinblatt M. E., Coblyn J. S., Fox D. A., Fraser P. A., Holdsworth D. E., Glass D. N., Trentham D. E. Efficacy of low-dose methotrexate in rheumatoid arthritis. N Engl J Med. 1985 Mar 28;312(13):818–822. doi: 10.1056/NEJM198503283121303. [DOI] [PubMed] [Google Scholar]
- Wijnands M. J., van't Hof M. A., van Leeuwen M. A., van Rijswijk M. H., van de Putte L. B., van Riel P. L. Long-term second-line treatment: a prospective drug survival study. Br J Rheumatol. 1992 Apr;31(4):253–258. doi: 10.1093/rheumatology/31.4.253. [DOI] [PubMed] [Google Scholar]
- Wilske K. R., Healey L. A. The need for aggressive therapy of rheumatoid arthritis. Rheum Dis Clin North Am. 1993 Feb;19(1):153–161. [PubMed] [Google Scholar]
- Wolfe F. Comparative usefulness of C-reactive protein and erythrocyte sedimentation rate in patients with rheumatoid arthritis. J Rheumatol. 1997 Aug;24(8):1477–1485. [PubMed] [Google Scholar]
- van der Heijde D. M., van 't Hof M., van Riel P. L., van de Putte L. B. Validity of single variables and indices to measure disease activity in rheumatoid arthritis. J Rheumatol. 1993 Mar;20(3):538–541. [PubMed] [Google Scholar]