Abstract
Background: When synovitis is proved, intra-articularly injected ß emitting radionuclides like yttrium-90 (90Y) are used to treat the inflamed synovium.
Objective: To study the viability, matrix production, and NO production during or after 90Y treatment of chondrocytes.
Methods: Monolayer, alginate, and explant cultures of primary bovine articular chondrocytes as well as synoviocytes were incubated with 0–3 MBq 90Y/ml medium for four days from culture day 3 onwards. Cell viability was demonstrated by light and electron microscopy or by trypan blue or ethidium bromide/fluorescein diacetate staining, membrane integrity by measurement of lactate dehydrogenase (LDH) activity in the culture supernatants. Biosynthetic activity was demonstrated by incorporation of [3H]proline and immunocytochemical staining of collagen type II. NO production was measured with the Griess reagent.
Results: In chondrocyte and synoviocyte monolayer cultures radiation caused a dose dependent increase in cell death and membrane destruction within four days. In alginate and explant cultures, where proliferation is low, no significantly increased LDH activity was seen, and cell viability was ∼100% for up to 14 days after irradiation. Collagen type II expression (alginate) and biosynthetic activity (alginate and explants) were decreased dose dependently while there was an increase in NO production. Light and electron microscopy data showed that five weeks after irradiation all cells in alginate and most cells in explants subjected to 3 MBq 90Y/ml were dead, whereas after lower amounts of irradiation several morphologically intact cells were found.
Conclusions: ß Irradiation may influence the long term maintenance of cartilage tissue or the aetiology of degenerative joint diseases.
Full Text
The Full Text of this article is available as a PDF (781.5 KB).
Figure 1 .
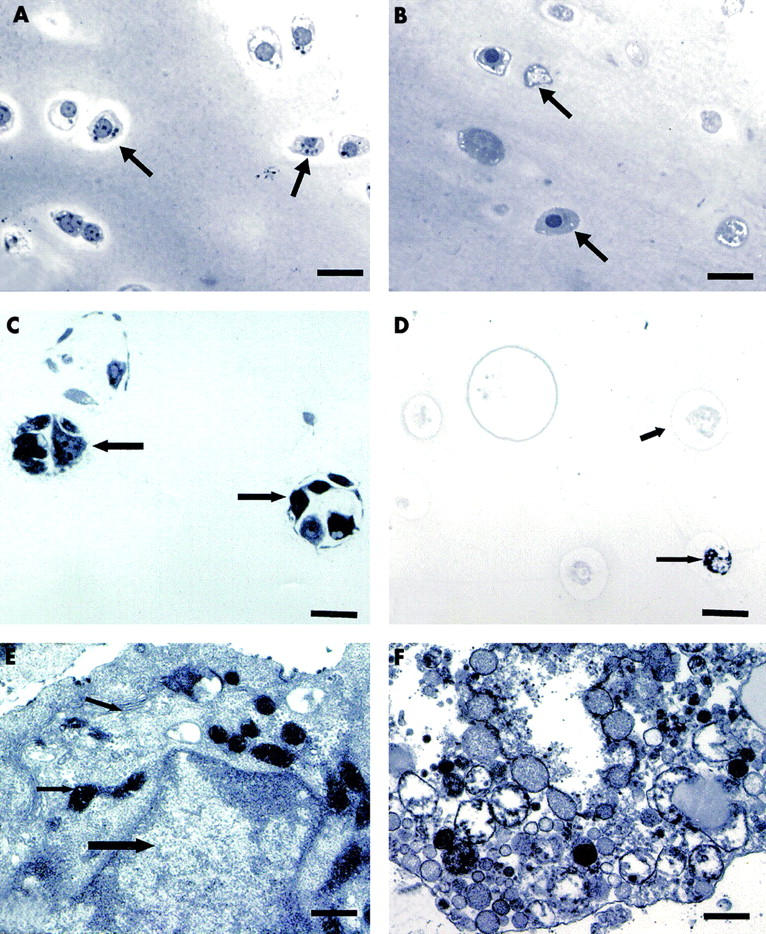
Semi-thin sections (A–D) and electron microscopy (E, F) of cartilage explants (A, B) and primary chondrocytes in alginate (C–F) five weeks after a four day period of ß irradiation indicating an induction of cell destruction. (A, C and E) Control cultures. Arrows demonstrate intact cells in A and C or intact organelles like the nucleus (large arrow) and the golgi apparatus and mitochondria (small arrows) in E. (B, D, and F) 3 MBq 90Y/ml. Arrows demonstrate damaged cells in B and D. In F remnants of organelles of a dead cell are visible. (A–D) bar=27 µm, (E, F) bar=0.2 µm.
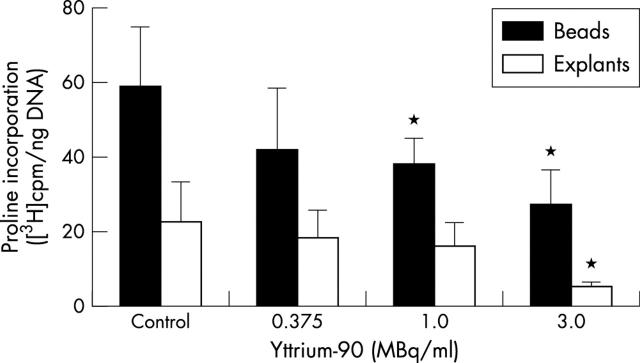
Figure 2 .
[3H]Proline incorporation in chondrocyte alginate cultures and cartilage explants two weeks after a four day period of 90Y irradiation. The bars show the dose dependent decrease of [3H]proline incorporation (p<0.004 by one way ANOVA) related to non-irradiated control cultures. Mean values (SD); n=6 (explants n=3); *p<0.05 v control by two tailed Student's t test.
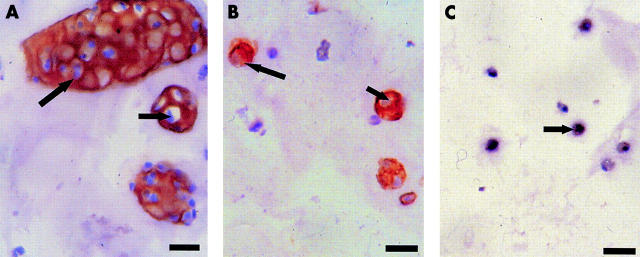
Figure 3 .
Immunocytochemical staining of collagen type II on cultures of chondrocytes in alginate beads two weeks after a four day period of 90Y irradiation. The figure shows the dose dependent decrease of collagen type II expression (brown staining; arrows demonstrate cells) compared with (A) the non-irradiated control; (B) 0.375 MBq; (C) 1 MBq 90Y. Bar=15.38 µm
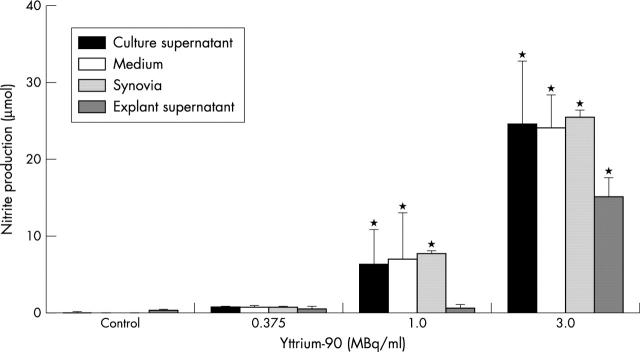
Figure 4 .
NO accumulation in supernatants of seven day old chondrocyte alginate and explant cultures, human synovial fluid (synovia) samples, and cell-free culture medium during four days of 90Y irradiation. The bars show the dose dependent increase of NO accumulation in the different systems. Mean values (SD); n=6 (explants n=3); *p<0.001 v control by two tailed Student's t test; p<0.001 by one way ANOVA.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bridgman J. F., Bruckner F., Eisen V., Tucker A., Bleehen N. M. Irradiation of the synovium in the treatment of rheumatoid arthritis. Q J Med. 1973 Apr;42(166):357–367. [PubMed] [Google Scholar]
- Cao M., Westerhausen-Larson A., Niyibizi C., Kavalkovich K., Georgescu H. I., Rizzo C. F., Hebda P. A., Stefanovic-Racic M., Evans C. H. Nitric oxide inhibits the synthesis of type-II collagen without altering Col2A1 mRNA abundance: prolyl hydroxylase as a possible target. Biochem J. 1997 May 15;324(Pt 1):305–310. doi: 10.1042/bj3240305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruz-Esteban C., Wilke W. S. Innovative treatment approaches for rheumatoid arthritis. Non-surgical synovectomy. Baillieres Clin Rheumatol. 1995 Nov;9(4):787–801. doi: 10.1016/s0950-3579(05)80314-0. [DOI] [PubMed] [Google Scholar]
- Delbarre F., Cayla J., Menkes C., Aignan M., Roucayrol J. C., Ingrand J. La synoviorthèse par les radio-isotopes. Presse Med. 1968 May 4;76(22):1045–1050. [PubMed] [Google Scholar]
- Deutsch E., Brodack J. W., Deutsch K. F. Radiation synovectomy revisited. Eur J Nucl Med. 1993 Nov;20(11):1113–1127. doi: 10.1007/BF00173494. [DOI] [PubMed] [Google Scholar]
- Domm C., Schünke M., Christesen K., Kurz B. Redifferentiation of dedifferentiated bovine articular chondrocytes in alginate culture under low oxygen tension. Osteoarthritis Cartilage. 2002 Jan;10(1):13–22. doi: 10.1053/joca.2001.0477. [DOI] [PubMed] [Google Scholar]
- Eckstein F., Winzheimer M., Westhoff J., Schnier M., Haubner M., Englmeier K. H., Reiser M., Putz R. Quantitative relationships of normal cartilage volumes of the human knee joint--assessment by magnetic resonance imaging. Anat Embryol (Berl) 1998 May;197(5):383–390. doi: 10.1007/s004290050149. [DOI] [PubMed] [Google Scholar]
- Gratz S., Göbel D., Becker W. Radiosynoviorthese bei entzündlichen Gelenkerkrankungen. Orthopade. 2000 Feb;29(2):164–170. doi: 10.1007/s001320050026. [DOI] [PubMed] [Google Scholar]
- Guaydier-Souquieres C., Beguin J., Ollivier D., Loyau G. Knee arthroscopy after yttrium or osmic acid injection. Arthroscopy. 1989;5(1):70–75. doi: 10.1016/0749-8063(89)90096-0. [DOI] [PubMed] [Google Scholar]
- Hagena F. W. Die Radio-Synoviorthese mit Yttrium 90 am Kniegelenk bei chronischer Polyarthritis. Fortschr Med. 1982 Sep 23;100(36):1673–1677. [PubMed] [Google Scholar]
- Hashimoto S., Takahashi K., Amiel D., Coutts R. D., Lotz M. Chondrocyte apoptosis and nitric oxide production during experimentally induced osteoarthritis. Arthritis Rheum. 1998 Jul;41(7):1266–1274. doi: 10.1002/1529-0131(199807)41:7<1266::AID-ART18>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- Heuft-Dorenbosch L. L., de Vet H. C., van der Linden S. Yttrium radiosynoviorthesis in the treatment of knee arthritis in rheumatoid arthritis: a systematic review. Ann Rheum Dis. 2000 Aug;59(8):583–586. doi: 10.1136/ard.59.8.583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ioannidis N., Kurz B., Hansen U., Schünke M. Influence of fulvic acid on the collagen secretion of bovine chondrocytes in vitro. Cell Tissue Res. 1999 Jul;297(1):141–147. doi: 10.1007/s004410051341. [DOI] [PubMed] [Google Scholar]
- Johnson L. S., Yanch J. C., Shortkroff S., Barnes C. L., Spitzer A. I., Sledge C. B. Beta-particle dosimetry in radiation synovectomy. Eur J Nucl Med. 1995 Sep;22(9):977–988. doi: 10.1007/BF00808408. [DOI] [PubMed] [Google Scholar]
- Kampen W. U., Brenner W., Kroeger S., Sawula J. A., Bohuslavizki K. H., Henze E. Long-term results of radiation synovectomy: a clinical follow-up study. Nucl Med Commun. 2001 Feb;22(2):239–246. doi: 10.1097/00006231-200102000-00017. [DOI] [PubMed] [Google Scholar]
- Kerschbaumer F., Bauer R., Falser N., Altmann H. Effects and side effects of radiosynovectomy with Yttrium 90 on rheumatic joint cartilage. Arch Orthop Trauma Surg. 1979 Jan 29;93(2):95–102. doi: 10.1007/BF00389679. [DOI] [PubMed] [Google Scholar]
- Kerschbaumer F., Kandziora F., Herresthal J., Hertel A., Hör G. Synovektomie und Synoviorthese als Kombinationstherapie bei rheumatoider Arthritis. Orthopade. 1998 Mar;27(3):188–196. doi: 10.1007/PL00003490. [DOI] [PubMed] [Google Scholar]
- Kurz B., Jin M., Patwari P., Cheng D. M., Lark M. W., Grodzinsky A. J. Biosynthetic response and mechanical properties of articular cartilage after injurious compression. J Orthop Res. 2001 Nov;19(6):1140–1146. doi: 10.1016/S0736-0266(01)00033-X. [DOI] [PubMed] [Google Scholar]
- Lotz M. The role of nitric oxide in articular cartilage damage. Rheum Dis Clin North Am. 1999 May;25(2):269–282. doi: 10.1016/s0889-857x(05)70067-3. [DOI] [PubMed] [Google Scholar]
- Menkes C. J. Is there a place for chemical and radiation synovectomy in rheumatic diseases? Rheumatol Rehabil. 1979 May;18(2):65–77. doi: 10.1093/rheumatology/18.2.65. [DOI] [PubMed] [Google Scholar]
- Myers S. L., Slowman S. D., Brandt K. D. Radiation synovectomy stimulates glycosaminoglycan synthesis by normal articular cartilage. J Lab Clin Med. 1989 Jul;114(1):27–35. [PubMed] [Google Scholar]
- Pavelka K., Meier-Ruge W., Müller W., Fridrich R. Histological study of effects of colloidal 90 yttrium on knee joint tissues of rabbits. Ann Rheum Dis. 1975 Feb;34(1):64–69. doi: 10.1136/ard.34.1.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rau R., Schütte H. Ergebnisse der Radiosynoviorthese mit Yttrium 90 bei chronischen Synovitiden--Eine prospektive Langzeituntersuchung. Teil I: Gesamtergebnis und Einfluss lokaler Faktoren. Z Rheumatol. 1983 Sep-Oct;42(5):265–270. [PubMed] [Google Scholar]
- Sadani G. R., Nadkarni G. D. Changes in lipid peroxide levels and the activity of reactive oxygen scavenging systems in thyroid tissue after exposure to radioactive iodine in rats. Thyroid. 1997 Dec;7(6):937–941. doi: 10.1089/thy.1997.7.937. [DOI] [PubMed] [Google Scholar]
- Sledge C. B., Atcher R. W., Shortkroff S., Anderson R. J., Bloomer W. D., Hurson B. J. Intra-articular radiation synovectomy. Clin Orthop Relat Res. 1984 Jan-Feb;(182):37–40. [PubMed] [Google Scholar]
- Takahashi K., Hashimoto S., Kubo T., Hirasawa Y., Lotz M., Amiel D. Effect of hyaluronan on chondrocyte apoptosis and nitric oxide production in experimentally induced osteoarthritis. J Rheumatol. 2000 Jul;27(7):1713–1720. [PubMed] [Google Scholar]
- Taylor W. J., Corkill M. M., Rajapaske C. N. A retrospective review of yttrium-90 synovectomy in the treatment of knee arthritis. Br J Rheumatol. 1997 Oct;36(10):1100–1105. doi: 10.1093/rheumatology/36.10.1100. [DOI] [PubMed] [Google Scholar]
- Verin V., Popowski Y., Bochaton-Piallat M. L., Belenger J., Urban P., Neuville P., Redard M., Costa M., Celetta G., Gabbiani G. Intraarterial beta irradiation induces smooth muscle cell apoptosis and reduces medial cellularity in a hypercholesterolemic rabbit restenosis model. Int J Radiat Oncol Biol Phys. 2000 Feb 1;46(3):661–670. doi: 10.1016/s0360-3016(99)00426-5. [DOI] [PubMed] [Google Scholar]
- Wagener P., Münch H., Junker D. Szintigraphische Untersuchungen zur Gonadenbelastung bei Radiosynoviorthesen des Kniegelenkes mit Yttrium-90. Z Rheumatol. 1988 Jul-Aug;47(4):201–204. [PubMed] [Google Scholar]
- Wang B., Takeda H., Gao W. M., Zhou X. Y., Odaka T., Ohyama H., Yamada T., Hayata I. Induction of apoptosis by beta radiation from tritium compounds in mouse embryonic brain cells. Health Phys. 1999 Jul;77(1):16–23. doi: 10.1097/00004032-199907000-00005. [DOI] [PubMed] [Google Scholar]
- Webb F. W., Lowe J., Bluestone R. Uptake of colloidal radioactive yttrium by synovial membrane. Ann Rheum Dis. 1969 May;28(3):300–302. doi: 10.1136/ard.28.3.300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de la Chapelle A., Rekonen A., Oka M., Ruotsi A. Chromosome damage after intra-articular injections of radioactive yttrium. Effect of immobilization on the biological dose. Ann Rheum Dis. 1972 Nov;31(6):508–512. doi: 10.1136/ard.31.6.508. [DOI] [PMC free article] [PubMed] [Google Scholar]