Abstract
Methods: 15 patients with active PsA who did not respond to DMARDs were randomly allocated to receive fludarabine every four weeks or placebo. Primary outcomes were the proportion of patients who met the ACR20 and the psoriatic arthritis response criteria (PsARC) at 16 weeks. Secondary outcomes were changes in tender or swollen joint counts and scores of the psoriasis area and severity index (PASI). Phenotypic analysis of peripheral blood mononuclear cells (PBMC), synovial immunohistochemistry, and functional analysis of PBMC were used to determine the immunomodulatory effects of fludarabine.
Results: At 16 weeks the ACR20 criteria were met by 3/7 (43%) fludarabine treated v 0/8 placebo treated patients (p=0.08); the PsARC was achieved by 4/7 (57%) fludarabine treated v 2/8 (25%) placebo treated patients; and 3/7 (43%) fludarabine treated v 0/7 placebo treated patients had ⩾20% improvement in the PASI. Marked peripheral lymphopenia involving naive (CD4+ CD45RA+) and memory (CD4+ CD45RO+) T cells, CD8+ T cells, and B cells was seen in fludarabine treated patients.
Conclusions: In PsA fludarabine induces significant peripheral, but modest, synovial lymphopenia, and a trend towards improved clinical response.
Full Text
The Full Text of this article is available as a PDF (315.3 KB).
Figure 1 .
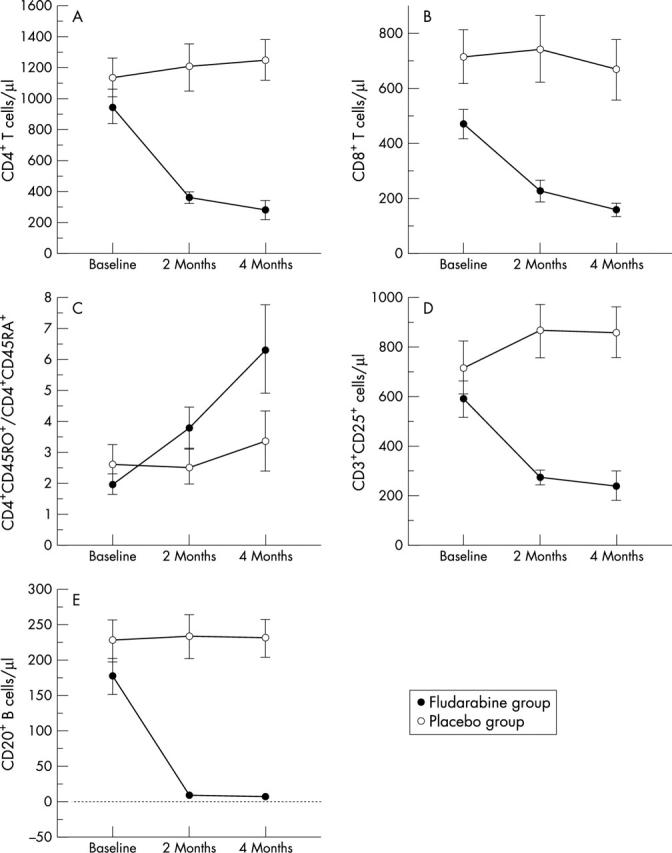
Effects of fludarabine on peripheral blood lymphocyte population. Mean (SEM) of the following at baseline, two months, and four months were plotted: numbers of (A) CD4+ T cells and (B) CD8+ T cells; (C) the ratio of CD4+ CD45RO+/CD4+ CD45RA+ cells; numbers of (D) CD25 expressing CD3+ cells and (E) CD20+ cells. Data were obtained for both the fludarabine and placebo groups.
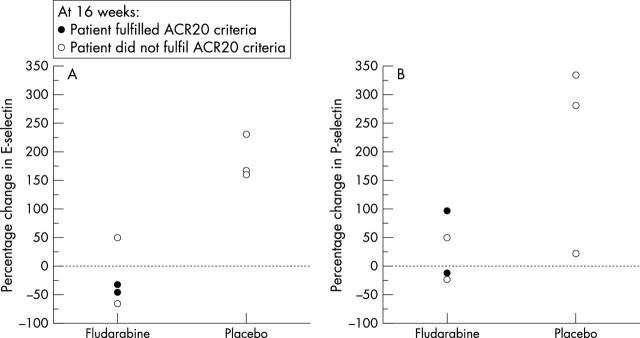
Figure 2 .
Effects of fludarabine on the activation status of lymphocytes and endothelial cells. Percentage changes in the proportion of (A) E-selectin and (B) P-selectin positive vessels in seven pairs of synovial biopsy specimens.