Full Text
The Full Text of this article is available as a PDF (494.9 KB).
Figure 1 .
Ductal cannulation of submandibular glands of a rat by using custom made cannulas. A maximal volume of 50 µl (mouse) or 200 µl (rat) of gene transfer vector can be delivered in a retrograde fashion using 3/10 ml insulin syringes connected to the cannulas. Arrows indicate the cannulas (see Baum et al24 ). Photo courtesy of Dr L Baccaglini.
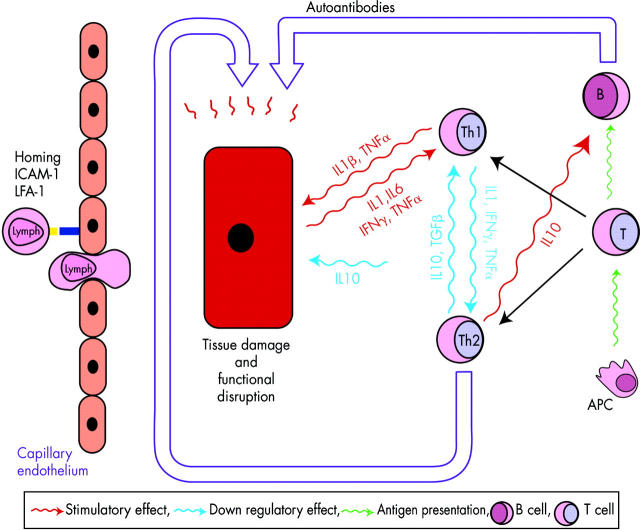
Figure 2 .
Schematic drawing of a salivary gland with a high endothelial venule, representing the lymphocyte traffic, the role of intercellular adhesion molecule-1 (ICAM-1)/leucocyte function associated antigen-1 (LFA-1), the formation of Th1- and Th2-like lymphocytes, and the key regulatory cytokines and their interactions in SS. The imbalance in Th1-like cells, producing proinflammatory cytokines, and Th2- like cells, results in local inflammation. The T-B cell interaction represents a pathway leading to autoantibody formation. This inflammatory microenvironment might result in tissue damage and disruption of secretory function. Also, note that the salivary gland epithelial cell can function as an antigen presenting cell (APC). Th1, T helper 1-like lymphocytes; Th2, T helper 2-like lymphocytes; Lymph, lymphocytes.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abbas A. K., Murphy K. M., Sher A. Functional diversity of helper T lymphocytes. Nature. 1996 Oct 31;383(6603):787–793. doi: 10.1038/383787a0. [DOI] [PubMed] [Google Scholar]
- Adamson T. C., 3rd, Fox R. I., Frisman D. M., Howell F. V. Immunohistologic analysis of lymphoid infiltrates in primary Sjogren's syndrome using monoclonal antibodies. J Immunol. 1983 Jan;130(1):203–208. [PubMed] [Google Scholar]
- BLOCH K. J., BUCHANAN W. W., WOHL M. J., BUNIM J. J. SJOEGREN'S SYNDROME. A CLINICAL, PATHOLOGICAL, AND SEROLOGICAL STUDY OF SIXTY-TWO CASES. Medicine (Baltimore) 1965 May;44:187–231. [PubMed] [Google Scholar]
- Bacman S., Perez Leiros C., Sterin-Borda L., Hubscher O., Arana R., Borda E. Autoantibodies against lacrimal gland M3 muscarinic acetylcholine receptors in patients with primary Sjögren's syndrome. Invest Ophthalmol Vis Sci. 1998 Jan;39(1):151–156. [PubMed] [Google Scholar]
- Bacman S., Sterin-Borda L., Camusso J. J., Arana R., Hubscher O., Borda E. Circulating antibodies against rat parotid gland M3 muscarinic receptors in primary Sjögren's syndrome. Clin Exp Immunol. 1996 Jun;104(3):454–459. doi: 10.1046/j.1365-2249.1996.42748.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bathon J. M., Martin R. W., Fleischmann R. M., Tesser J. R., Schiff M. H., Keystone E. C., Genovese M. C., Wasko M. C., Moreland L. W., Weaver A. L. A comparison of etanercept and methotrexate in patients with early rheumatoid arthritis. N Engl J Med. 2000 Nov 30;343(22):1586–1593. doi: 10.1056/NEJM200011303432201. [DOI] [PubMed] [Google Scholar]
- Baum B. J., O'Connell B. C. In vivo gene transfer to salivary glands. Crit Rev Oral Biol Med. 1999;10(3):276–283. doi: 10.1177/10454411990100030201. [DOI] [PubMed] [Google Scholar]
- Baum Bruce J., Wellner Robert B., Zheng Changyu. Gene transfer to salivary glands. Int Rev Cytol. 2002;213:93–146. doi: 10.1016/s0074-7696(02)13013-0. [DOI] [PubMed] [Google Scholar]
- Braddon V. R., Chiorini J. A., Wang S., Kotin R. M., Baum B. J. Adenoassociated virus-mediated transfer of a functional water channel into salivary epithelial cells in vitro and in vivo. Hum Gene Ther. 1998 Dec 10;9(18):2777–2785. doi: 10.1089/hum.1998.9.18-2777. [DOI] [PubMed] [Google Scholar]
- Cortes J., Kurzrock R. Interleukin-10 in non-Hodgkin's lymphoma. Leuk Lymphoma. 1997 Jul;26(3-4):251–259. doi: 10.3109/10428199709051774. [DOI] [PubMed] [Google Scholar]
- Datta S. K., Kalled S. L. CD40-CD40 ligand interaction in autoimmune disease. Arthritis Rheum. 1997 Oct;40(10):1735–1745. doi: 10.1002/art.1780401002. [DOI] [PubMed] [Google Scholar]
- Edwards J. C., Cambridge G. Sustained improvement in rheumatoid arthritis following a protocol designed to deplete B lymphocytes. Rheumatology (Oxford) 2001 Feb;40(2):205–211. doi: 10.1093/rheumatology/40.2.205. [DOI] [PubMed] [Google Scholar]
- Evans C. H., Ghivizzani S. C., Kang R., Muzzonigro T., Wasko M. C., Herndon J. H., Robbins P. D. Gene therapy for rheumatic diseases. Arthritis Rheum. 1999 Jan;42(1):1–16. doi: 10.1002/1529-0131(199901)42:1<1::AID-ANR1>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- Ferraccioli G. F., De Vita S. Cytokine expression in the salivary glands of Sjögren's syndrome patients in relation to tissue infiltration and lymphoepithelial lesions. Arthritis Rheum. 1997 May;40(5):987–990. doi: 10.1002/art.1780400537. [DOI] [PubMed] [Google Scholar]
- Fox P. C., Brennan M., Di Sun P. Cytokine expression in human labial minor salivary gland epithelial cells in health and disease. Arch Oral Biol. 1999 May;44 (Suppl 1):S49–S52. doi: 10.1016/s0003-9969(99)90018-3. [DOI] [PubMed] [Google Scholar]
- Fox P. C., Datiles M., Atkinson J. C., Macynski A. A., Scott J., Fletcher D., Valdez I. H., Kurrasch R. H., Delapenha R., Jackson W. Prednisone and piroxicam for treatment of primary Sjögren's syndrome. Clin Exp Rheumatol. 1993 Mar-Apr;11(2):149–156. [PubMed] [Google Scholar]
- Fox P. C., Grisius M. M., Bermudez D. K., Sun D. Cytokine mRNA expression in labial salivary glands and cytokine secretion in parotid saliva in Sjögren's syndrome. Adv Exp Med Biol. 1998;438:909–915. doi: 10.1007/978-1-4615-5359-5_129. [DOI] [PubMed] [Google Scholar]
- Fox P. C., van der Ven P. F., Baum B. J., Mandel I. D. Pilocarpine for the treatment of xerostomia associated with salivary gland dysfunction. Oral Surg Oral Med Oral Pathol. 1986 Mar;61(3):243–248. doi: 10.1016/0030-4220(86)90369-5. [DOI] [PubMed] [Google Scholar]
- Fox R. I., Kang H. I., Ando D., Abrams J., Pisa E. Cytokine mRNA expression in salivary gland biopsies of Sjögren's syndrome. J Immunol. 1994 Jun 1;152(11):5532–5539. [PubMed] [Google Scholar]
- Fox R. I., Konttinen Y., Fisher A. Use of muscarinic agonists in the treatment of Sjögren's syndrome. Clin Immunol. 2001 Dec;101(3):249–263. doi: 10.1006/clim.2001.5128. [DOI] [PubMed] [Google Scholar]
- Fox R. I., Michelson P., Casiano C. A., Hayashi J., Stern M. Sjögren's syndrome. Clin Dermatol. 2000 Sep-Oct;18(5):589–600. doi: 10.1016/s0738-081x(00)00135-8. [DOI] [PubMed] [Google Scholar]
- Furukawa F. Animal models of cutaneous lupus erythematosus and lupus erythematosus photosensitivity. Lupus. 1997;6(2):193–202. doi: 10.1177/096120339700600215. [DOI] [PubMed] [Google Scholar]
- Go N. F., Castle B. E., Barrett R., Kastelein R., Dang W., Mosmann T. R., Moore K. W., Howard M. Interleukin 10, a novel B cell stimulatory factor: unresponsiveness of X chromosome-linked immunodeficiency B cells. J Exp Med. 1990 Dec 1;172(6):1625–1631. doi: 10.1084/jem.172.6.1625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groom Joanna, Kalled Susan L., Cutler Anne H., Olson Carl, Woodcock Stephen A., Schneider Pascal, Tschopp Jurg, Cachero Teresa G., Batten Marcel, Wheway Julie. Association of BAFF/BLyS overexpression and altered B cell differentiation with Sjögren's syndrome. J Clin Invest. 2002 Jan;109(1):59–68. doi: 10.1172/JCI14121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hacein-Bey-Abina Salima, von Kalle Christof, Schmidt Manfred, Le Deist Françoise, Wulffraat Nicolas, McIntyre Elisabeth, Radford Isabelle, Villeval Jean-Luc, Fraser Christopher C., Cavazzana-Calvo Marina. A serious adverse event after successful gene therapy for X-linked severe combined immunodeficiency. N Engl J Med. 2003 Jan 16;348(3):255–256. doi: 10.1056/NEJM200301163480314. [DOI] [PubMed] [Google Scholar]
- Hamano H., Saito I., Haneji N., Mitsuhashi Y., Miyasaka N., Hayashi Y. Expressions of cytokine genes during development of autoimmune sialadenitis in MRL/lpr mice. Eur J Immunol. 1993 Oct;23(10):2387–2391. doi: 10.1002/eji.1830231002. [DOI] [PubMed] [Google Scholar]
- Haneji N., Hamano H., Yanagi K., Hayashi Y. A new animal model for primary Sjögren's syndrome in NFS/sld mutant mice. J Immunol. 1994 Sep 15;153(6):2769–2777. [PubMed] [Google Scholar]
- Haneji N., Nakamura T., Takio K., Yanagi K., Higashiyama H., Saito I., Noji S., Sugino H., Hayashi Y. Identification of alpha-fodrin as a candidate autoantigen in primary Sjögren's syndrome. Science. 1997 Apr 25;276(5312):604–607. doi: 10.1126/science.276.5312.604. [DOI] [PubMed] [Google Scholar]
- Hayashi Y., Haneji N., Hamano H. Pathogenesis of Sjögren's syndrome-like autoimmune lesions in MRL/lpr mice. Pathol Int. 1994 Aug;44(8):559–568. doi: 10.1111/j.1440-1827.1994.tb01716.x. [DOI] [PubMed] [Google Scholar]
- Hayashi Y., Haneji N., Hamano H., Yanagi K., Takahashi M., Ishimaru N. Effector mechanism of experimental autoimmune sialadenitis in the mouse model for primary Sjögren's syndrome. Cell Immunol. 1996 Aug 1;171(2):217–225. doi: 10.1006/cimm.1996.0196. [DOI] [PubMed] [Google Scholar]
- Hoque A. T., Baccaglini L., Baum B. J. Hydroxychloroquine enhances the endocrine secretion of adenovirus-directed growth hormone from rat submandibular glands in vivo. Hum Gene Ther. 2001 Jul 1;12(10):1333–1341. doi: 10.1089/104303401750270986. [DOI] [PubMed] [Google Scholar]
- Humphreys-Beher M. G., Brayer J., Yamachika S., Peck A. B., Jonsson R. An alternative perspective to the immune response in autoimmune exocrinopathy: induction of functional quiescence rather than destructive autoaggression. Scand J Immunol. 1999 Jan;49(1):7–10. doi: 10.1046/j.1365-3083.1999.00490.x. [DOI] [PubMed] [Google Scholar]
- Humphreys-Beher M. G., Hu Y., Nakagawa Y., Wang P. L., Purushotham K. R. Utilization of the non-obese diabetic (NOD) mouse as an animal model for the study of secondary Sjögren's syndrome. Adv Exp Med Biol. 1994;350:631–636. doi: 10.1007/978-1-4615-2417-5_105. [DOI] [PubMed] [Google Scholar]
- Ioannidis John P. A., Vassiliou Vassilios A., Moutsopoulos Haralampos M. Long-term risk of mortality and lymphoproliferative disease and predictive classification of primary Sjögren's syndrome. Arthritis Rheum. 2002 Mar;46(3):741–747. doi: 10.1002/art.10221. [DOI] [PubMed] [Google Scholar]
- Itoh N., Yonehara S., Ishii A., Yonehara M., Mizushima S., Sameshima M., Hase A., Seto Y., Nagata S. The polypeptide encoded by the cDNA for human cell surface antigen Fas can mediate apoptosis. Cell. 1991 Jul 26;66(2):233–243. doi: 10.1016/0092-8674(91)90614-5. [DOI] [PubMed] [Google Scholar]
- Jonsson R., Tarkowski A., Bäckman K., Holmdahl R., Klareskog L. Sialadenitis in the MRL-l mouse: morphological and immunohistochemical characterization of resident and infiltrating cells. Immunology. 1987 Apr;60(4):611–616. [PMC free article] [PubMed] [Google Scholar]
- Kagami H., Atkinson J. C., Michalek S. M., Handelman B., Yu S., Baum B. J., O'Connell B. Repetitive adenovirus administration to the parotid gland: role of immunological barriers and induction of oral tolerance. Hum Gene Ther. 1998 Feb 10;9(3):305–313. doi: 10.1089/hum.1998.9.3-305. [DOI] [PubMed] [Google Scholar]
- Kay M. A., Glorioso J. C., Naldini L. Viral vectors for gene therapy: the art of turning infectious agents into vehicles of therapeutics. Nat Med. 2001 Jan;7(1):33–40. doi: 10.1038/83324. [DOI] [PubMed] [Google Scholar]
- Kessler H. S. A laboratory model for Sjögren's syndrome. Am J Pathol. 1968 Mar;52(3):671–685. [PMC free article] [PubMed] [Google Scholar]
- Kong L., Ogawa N., McGuff H. S., Nakabayashi T., Sakata K. M., Masago R., Vela-Roch N., Talal N., Dang H. Bcl-2 family expression in salivary glands from patients with primary Sjögren's syndrome: involvement of Bax in salivary gland destruction. Clin Immunol Immunopathol. 1998 Aug;88(2):133–141. doi: 10.1006/clin.1998.4556. [DOI] [PubMed] [Google Scholar]
- Kong L., Ogawa N., Nakabayashi T., Liu G. T., D'Souza E., McGuff H. S., Guerrero D., Talal N., Dang H. Fas and Fas ligand expression in the salivary glands of patients with primary Sjögren's syndrome. Arthritis Rheum. 1997 Jan;40(1):87–97. doi: 10.1002/art.1780400113. [DOI] [PubMed] [Google Scholar]
- Koopman W. J., Gay S. The MRL-lpr/lpr mouse. A model for the study of rheumatoid arthritis. Scand J Rheumatol Suppl. 1988;75:284–289. doi: 10.3109/03009748809096780. [DOI] [PubMed] [Google Scholar]
- Kruize A. A., Hené R. J., Kallenberg C. G., van Bijsterveld O. P., van der Heide A., Kater L., Bijlsma J. W. Hydroxychloroquine treatment for primary Sjögren's syndrome: a two year double blind crossover trial. Ann Rheum Dis. 1993 May;52(5):360–364. doi: 10.1136/ard.52.5.360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Hir M., Martin M., Haas C. A syndrome resembling human systemic sclerosis (scleroderma) in MRL/lpr mice lacking interferon-gamma (IFN-gamma) receptor (MRL/lprgammaR-/-). Clin Exp Immunol. 1999 Feb;115(2):281–287. doi: 10.1046/j.1365-2249.1999.00808.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leiden J. M. Gene therapy--promise, pitfalls, and prognosis. N Engl J Med. 1995 Sep 28;333(13):871–873. doi: 10.1056/NEJM199509283331310. [DOI] [PubMed] [Google Scholar]
- Linsley P. S., Wallace P. M., Johnson J., Gibson M. G., Greene J. L., Ledbetter J. A., Singh C., Tepper M. A. Immunosuppression in vivo by a soluble form of the CTLA-4 T cell activation molecule. Science. 1992 Aug 7;257(5071):792–795. doi: 10.1126/science.1496399. [DOI] [PubMed] [Google Scholar]
- Lund T., O'Reilly L., Hutchings P., Kanagawa O., Simpson E., Gravely R., Chandler P., Dyson J., Picard J. K., Edwards A. Prevention of insulin-dependent diabetes mellitus in non-obese diabetic mice by transgenes encoding modified I-A beta-chain or normal I-E alpha-chain. Nature. 1990 Jun 21;345(6277):727–729. doi: 10.1038/345727a0. [DOI] [PubMed] [Google Scholar]
- Makino S., Kunimoto K., Muraoka Y., Mizushima Y., Katagiri K., Tochino Y. Breeding of a non-obese, diabetic strain of mice. Jikken Dobutsu. 1980 Jan;29(1):1–13. doi: 10.1538/expanim1978.29.1_1. [DOI] [PubMed] [Google Scholar]
- Mariette X., Roux S., Zhang J., Bengoufa D., Lavie F., Zhou T., Kimberly R. The level of BLyS (BAFF) correlates with the titre of autoantibodies in human Sjögren's syndrome. Ann Rheum Dis. 2003 Feb;62(2):168–171. doi: 10.1136/ard.62.2.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mariette X., Sibilia J., Roux S., Meignin V., Janin A. A new defensive mechanism to prevent apoptosis in salivary ductal cells from patients with Sjögren's syndrome: over-expression of p53 and p21. Rheumatology (Oxford) 2002 Jan;41(1):96–99. doi: 10.1093/rheumatology/41.1.96. [DOI] [PubMed] [Google Scholar]
- Marshall E. Gene therapy. Viral vectors still pack surprises. Science. 2001 Nov 23;294(5547):1640–1640. doi: 10.1126/science.294.5547.1640. [DOI] [PubMed] [Google Scholar]
- Martens P. B., Pillemer S. R., Jacobsson L. T., O'Fallon W. M., Matteson E. L. Survivorship in a population based cohort of patients with Sjögren's syndrome, 1976-1992. J Rheumatol. 1999 Jun;26(6):1296–1300. [PubMed] [Google Scholar]
- Mastrangeli A., O'Connell B., Aladib W., Fox P. C., Baum B. J., Crystal R. G. Direct in vivo adenovirus-mediated gene transfer to salivary glands. Am J Physiol. 1994 Jun;266(6 Pt 1):G1146–G1155. doi: 10.1152/ajpgi.1994.266.6.G1146. [DOI] [PubMed] [Google Scholar]
- Mitsias D. I., Tzioufas A. G., Veiopoulou C., Zintzaras E., Tassios I. K., Kogopoulou O., Moutsopoulos H. M., Thyphronitis G. The Th1/Th2 cytokine balance changes with the progress of the immunopathological lesion of Sjogren's syndrome. Clin Exp Immunol. 2002 Jun;128(3):562–568. doi: 10.1046/j.1365-2249.2002.01869.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore K. W., de Waal Malefyt R., Coffman R. L., O'Garra A. Interleukin-10 and the interleukin-10 receptor. Annu Rev Immunol. 2001;19:683–765. doi: 10.1146/annurev.immunol.19.1.683. [DOI] [PubMed] [Google Scholar]
- Morgan R. A., Anderson W. F. Human gene therapy. Annu Rev Biochem. 1993;62:191–217. doi: 10.1146/annurev.bi.62.070193.001203. [DOI] [PubMed] [Google Scholar]
- Mosmann T. R., Cherwinski H., Bond M. W., Giedlin M. A., Coffman R. L. Two types of murine helper T cell clone. I. Definition according to profiles of lymphokine activities and secreted proteins. J Immunol. 1986 Apr 1;136(7):2348–2357. [PubMed] [Google Scholar]
- Mosmann T. R., Coffman R. L. TH1 and TH2 cells: different patterns of lymphokine secretion lead to different functional properties. Annu Rev Immunol. 1989;7:145–173. doi: 10.1146/annurev.iy.07.040189.001045. [DOI] [PubMed] [Google Scholar]
- Moutsopoulos H. M. Sjögren's syndrome: autoimmune epithelitis. Clin Immunol Immunopathol. 1994 Aug;72(2):162–165. doi: 10.1006/clin.1994.1123. [DOI] [PubMed] [Google Scholar]
- Nagaraju K., Cox A., Casciola-Rosen L., Rosen A. Novel fragments of the Sjögren's syndrome autoantigens alpha-fodrin and type 3 muscarinic acetylcholine receptor generated during cytotoxic lymphocyte granule-induced cell death. Arthritis Rheum. 2001 Oct;44(10):2376–2386. doi: 10.1002/1529-0131(200110)44:10<2376::aid-art402>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- Nishikawa M., Huang L. Nonviral vectors in the new millennium: delivery barriers in gene transfer. Hum Gene Ther. 2001 May 20;12(8):861–870. doi: 10.1089/104303401750195836. [DOI] [PubMed] [Google Scholar]
- Ogawa N., Dang H., Kong L., Anaya J. M., Liu G. T., Talal N. Lymphocyte apoptosis and apoptosis-associated gene expression in Sjögren's syndrome. Arthritis Rheum. 1996 Nov;39(11):1875–1885. doi: 10.1002/art.1780391114. [DOI] [PubMed] [Google Scholar]
- Ohlsson M., Skarstein K., Bolstad A. I., Johannessen A. C., Jonsson R. Fas-induced apoptosis is a rare event in Sjögren's syndrome. Lab Invest. 2001 Jan;81(1):95–105. doi: 10.1038/labinvest.3780215. [DOI] [PubMed] [Google Scholar]
- Ohyama Y., Nakamura S., Matsuzaki G., Shinohara M., Hiroki A., Fujimura T., Yamada A., Itoh K., Nomoto K. Cytokine messenger RNA expression in the labial salivary glands of patients with Sjögren's syndrome. Arthritis Rheum. 1996 Aug;39(8):1376–1384. doi: 10.1002/art.1780390816. [DOI] [PubMed] [Google Scholar]
- Oxholm P., Daniels T. E., Bendtzen K. Cytokine expression in labial salivary glands from patients with primary Sjögren's syndrome. Autoimmunity. 1992;12(3):185–191. doi: 10.3109/08916939209148458. [DOI] [PubMed] [Google Scholar]
- Patel Y. I., McHugh N. J. Apoptosis-new clues to the pathogenesis of Sjögren's syndrome? Rheumatology (Oxford) 2000 Feb;39(2):119–121. doi: 10.1093/rheumatology/39.2.119. [DOI] [PubMed] [Google Scholar]
- Pedersen A. M., Dissing S., Fahrenkrug J., Hannibal J., Reibel J., Nauntofte B. Innervation pattern and Ca2+ signalling in labial salivary glands of healthy individuals and patients with primary Sjögren's syndrome (pSS). J Oral Pathol Med. 2000 Mar;29(3):97–109. doi: 10.1034/j.1600-0714.2000.290301.x. [DOI] [PubMed] [Google Scholar]
- Pillemer S. R., Matteson E. L., Jacobsson L. T., Martens P. B., Melton L. J., 3rd, O'Fallon W. M., Fox P. C. Incidence of physician-diagnosed primary Sjögren syndrome in residents of Olmsted County, Minnesota. Mayo Clin Proc. 2001 Jun;76(6):593–599. doi: 10.4065/76.6.593. [DOI] [PubMed] [Google Scholar]
- Robinson C. P., Yamachika S., Bounous D. I., Brayer J., Jonsson R., Holmdahl R., Peck A. B., Humphreys-Beher M. G. A novel NOD-derived murine model of primary Sjögren's syndrome. Arthritis Rheum. 1998 Jan;41(1):150–156. doi: 10.1002/1529-0131(199801)41:1<150::AID-ART18>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- Robinson C. P., Yamamoto H., Peck A. B., Humphreys-Beher M. G. Genetically programmed development of salivary gland abnormalities in the NOD (nonobese diabetic)-scid mouse in the absence of detectable lymphocytic infiltration: a potential trigger for sialoadenitis of NOD mice. Clin Immunol Immunopathol. 1996 Apr;79(1):50–59. doi: 10.1006/clin.1996.0050. [DOI] [PubMed] [Google Scholar]
- Saito I., Terauchi K., Shimuta M., Nishiimura S., Yoshino K., Takeuchi T., Tsubota K., Miyasaka N. Expression of cell adhesion molecules in the salivary and lacrimal glands of Sjogren's syndrome. J Clin Lab Anal. 1993;7(3):180–187. doi: 10.1002/jcla.1860070309. [DOI] [PubMed] [Google Scholar]
- Salgame P., Abrams J. S., Clayberger C., Goldstein H., Convit J., Modlin R. L., Bloom B. R. Differing lymphokine profiles of functional subsets of human CD4 and CD8 T cell clones. Science. 1991 Oct 11;254(5029):279–282. doi: 10.1126/science.254.5029.279. [DOI] [PubMed] [Google Scholar]
- Schnell M. A., Zhang Y., Tazelaar J., Gao G. P., Yu Q. C., Qian R., Chen S. J., Varnavski A. N., LeClair C., Raper S. E. Activation of innate immunity in nonhuman primates following intraportal administration of adenoviral vectors. Mol Ther. 2001 May;3(5 Pt 1):708–722. doi: 10.1006/mthe.2001.0330. [DOI] [PubMed] [Google Scholar]
- Sfikakis P. P., Mavrikakis M. Adhesion and lymphocyte costimulatory molecules in systemic rheumatic diseases. Clin Rheumatol. 1999;18(4):317–327. doi: 10.1007/s100670050109. [DOI] [PubMed] [Google Scholar]
- Shai Ela, Falk Haya, Honigman Alik, Panet Amos, Palmon Aaron. Gene transfer mediated by different viral vectors following direct cannulation of mouse submandibular salivary glands. Eur J Oral Sci. 2002 Jun;110(3):254–260. doi: 10.1034/j.1600-0722.2002.21200.x. [DOI] [PubMed] [Google Scholar]
- Shin S. S., Sheibani K., Fishleder A., Ben-Ezra J., Bailey A., Koo C. H., Burke J. S., Tubbs R., Rappaport H. Monocytoid B-cell lymphoma in patients with Sjögren's syndrome: a clinicopathologic study of 13 patients. Hum Pathol. 1991 May;22(5):422–430. doi: 10.1016/0046-8177(91)90126-a. [DOI] [PubMed] [Google Scholar]
- Skopouli F. N., Fox P. C., Galanopoulou V., Atkinson J. C., Jaffe E. S., Moutsopoulos H. M. T cell subpopulations in the labial minor salivary gland histopathologic lesion of Sjögren's syndrome. J Rheumatol. 1991 Feb;18(2):210–214. [PubMed] [Google Scholar]
- Steinfeld S. D., Demols P., Salmon I., Kiss R., Appelboom T. Infliximab in patients with primary Sjögren's syndrome: a pilot study. Arthritis Rheum. 2001 Oct;44(10):2371–2375. doi: 10.1002/1529-0131(200110)44:10<2371::aid-art401>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- Strömbeck B., Ekdahl C., Manthorpe R., Wikström I., Jacobsson L. Health-related quality of life in primary Sjögren's syndrome, rheumatoid arthritis and fibromyalgia compared to normal population data using SF-36. Scand J Rheumatol. 2000;29(1):20–28. doi: 10.1080/030097400750001761. [DOI] [PubMed] [Google Scholar]
- Sun D., Emmert-Buck M. R., Fox P. C. Differential cytokine mRNA expression in human labial minor salivary glands in primary Sjögren's syndrome. Autoimmunity. 1998;28(3):125–137. doi: 10.3109/08916939808996281. [DOI] [PubMed] [Google Scholar]
- Umetsu D. T., Jabara H. H., DeKruyff R. H., Abbas A. K., Abrams J. S., Geha R. S. Functional heterogeneity among human inducer T cell clones. J Immunol. 1988 Jun 15;140(12):4211–4216. [PubMed] [Google Scholar]
- Van Blokland Saskia C. A., Van Helden-Meeuwsen Cornelia G., Wierenga-Wolf Annet F., Tielemans Dennis, Drexhage Hemmo A., Van De Merwe Joop P., Homo-Delarche Françoise, Versnel Marjan A. Apoptosis and apoptosis-related molecules in the submandibular gland of the nonobese diabetic mouse model for Sjögren's syndrome: limited role for apoptosis in the development of sialoadenitis. Lab Invest. 2003 Jan;83(1):3–11. doi: 10.1097/01.lab.0000048721.21475.d1. [DOI] [PubMed] [Google Scholar]
- Vervoordeldonk M. J., Tak P. P. Gene therapy in rheumatic diseases. Best Pract Res Clin Rheumatol. 2001 Dec;15(5):771–788. doi: 10.1053/berh.2001.0193. [DOI] [PubMed] [Google Scholar]
- Waterman S. A., Gordon T. P., Rischmueller M. Inhibitory effects of muscarinic receptor autoantibodies on parasympathetic neurotransmission in Sjögren's syndrome. Arthritis Rheum. 2000 Jul;43(7):1647–1654. doi: 10.1002/1529-0131(200007)43:7<1647::AID-ANR31>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- Westermann J., Engelhardt B., Hoffmann J. C. Migration of T cells in vivo: molecular mechanisms and clinical implications. Ann Intern Med. 2001 Aug 21;135(4):279–295. doi: 10.7326/0003-4819-135-4-200108210-00013. [DOI] [PubMed] [Google Scholar]
- Witte T., Matthias T., Arnett F. C., Peter H. H., Hartung K., Sachse C., Wigand R., Braner A., Kalden J. R., Lakomek H. J. IgA and IgG autoantibodies against alpha-fodrin as markers for Sjögren's syndrome. Systemic lupus erythematosus. J Rheumatol. 2000 Nov;27(11):2617–2620. [PubMed] [Google Scholar]
- Wolff A., Scott J., Woods K., Fox P. C. An investigation of parotid gland function and histopathology in autoimmune disease-prone mice of different age groups. J Oral Pathol Med. 1991 Nov;20(10):486–489. doi: 10.1111/j.1600-0714.1991.tb00409.x. [DOI] [PubMed] [Google Scholar]
- Yamano S., Atkinson J. C., Baum B. J., Fox P. C. Salivary gland cytokine expression in NOD and normal BALB/c mice. Clin Immunol. 1999 Sep;92(3):265–275. doi: 10.1006/clim.1999.4759. [DOI] [PubMed] [Google Scholar]
- Yamano S., Scott D. E., Huang L. Y., Mikolajczyk M., Pillemer S. R., Chiorini J. A., Golding B., Baum B. J. Protection from experimental endotoxemia by a recombinant adeno-associated virus encoding interleukin 10. J Gene Med. 2001 Sep-Oct;3(5):450–457. doi: 10.1002/jgm.213. [DOI] [PubMed] [Google Scholar]
- Yamano Seiichi, Huang Li-Yun, Ding Chuantian, Chiorini John A., Goldsmith Corinne M., Wellner Robert B., Golding Basil, Kotin Robert M., Scott Dorothy E., Baum Bruce J. Recombinant adeno-associated virus serotype 2 vectors mediate stable interleukin 10 secretion from salivary glands into the bloodstream. Hum Gene Ther. 2002 Jan 20;13(2):287–298. doi: 10.1089/10430340252769806. [DOI] [PubMed] [Google Scholar]
- Zhao N., Liu D. P., Liang C. C. Hot topics in adeno-associated virus as a gene transfer vector. Mol Biotechnol. 2001 Nov;19(3):229–237. doi: 10.1385/MB:19:3:229. [DOI] [PubMed] [Google Scholar]
- Zheng C., Hoque A. T., Braddon V. R., Baum B. J., O'Connell B. C. Evaluation of salivary gland acinar and ductal cell-specific promoters in vivo with recombinant adenoviral vectors. Hum Gene Ther. 2001 Dec 10;12(18):2215–2223. doi: 10.1089/10430340152710559. [DOI] [PubMed] [Google Scholar]
- Zumla A., Mathur M., Stewart J., Wilkinson L., Isenberg D. T cell receptor expression in Sjögren's syndrome. Ann Rheum Dis. 1991 Oct;50(10):691–693. doi: 10.1136/ard.50.10.691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Roon J. A., van Roy J. L., Gmelig-Meyling F. H., Lafeber F. P., Bijlsma J. W. Prevention and reversal of cartilage degradation in rheumatoid arthritis by interleukin-10 and interleukin-4. Arthritis Rheum. 1996 May;39(5):829–835. doi: 10.1002/art.1780390516. [DOI] [PubMed] [Google Scholar]